Fungal Infections in Preterm Infants
- Author: David A Kaufman, MD; Chief Editor: Ted Rosenkrantz, MD more...
Updated: Jun 25, 2014
Introduction and Pathogenesis
Introduction
The risk for invasive fungal infections is high in very low birth weight (VLBW) infants (< 1500 g) and highest for infants born at the youngest gestational ages who survive past the immediate postnatal period.[1, 2] These immunocompromised infants usually require invasive therapies, such as central vascular catheters and endotracheal tubes, and are exposed to broad-spectrum antibiotics and parenteral nutrition. In addition, they occasionally receive postnatal steroids and gastric acid inhibitors. All of these factors place them at high risk for fungal infection. The incidence had been increasing in infants less than 1000 g with the resuscitation and survival of more and more infants prior to the studies of and broad institution of antifungal prophylaxis in high-risk preterm infants.[3, 4]Most fungal infections in preterm neonates are due to Candida species; a much smaller number of infections may be attributed Malassezia, Zygomycetes, or Aspergillus pathogens. Candida species are commensal organisms that colonize the skin and mucosal surfaces and adhere to catheter surfaces. Candidaalbicans and parapsilosis account for 80-90% of infections. Candida can invade the bloodstream and disseminate in these infants because of their immature immune systems, complicated by the inevitable need to compromise their developing skin and mucosal membrane barrier defenses. For these reasons, fungal infections are often difficult to eradicate in the preterm infant.
Although these immunocompromised infants are at increased risk during most of their hospital stay, they are at the highest risk of acquiring invasive fungal infections during the first weeks of life, when the most invasive therapies are performed and remain in place. Although an index of suspicion must always remain high, infection control, prophylaxis, and aggressive treatment (antifungal therapy and central catheter removal) during this period have the greatest potential to improve the outcome of this population.
Pathogenesis
The pathogenesis of fungal infections in preterm infants involves adherence, colonization, and dissemination (as is shown in the image below).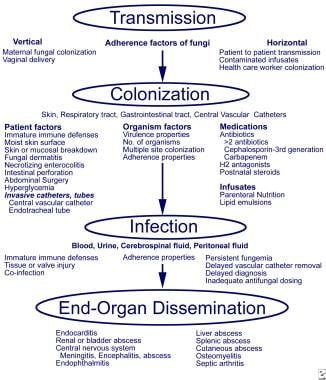 Pathogenesis and invasive fungal infections in very low birth weight infants. From Kaufman and Fairchild 2004, with permission.
Pathogenesis and invasive fungal infections in very low birth weight infants. From Kaufman and Fairchild 2004, with permission. The preterm infant is immunocompromised and frequently exposed to broad-spectrum antibacterial medications. Investigators have studied the effect of steroids and antibiotics in mice orally inoculated with Candida albicans to mimic conditions in the preterm infant.[5] Antibiotic treatment alone led to increased Candida colonization but did not affect dissemination. When dexamethasone was added to the antibiotic regimen (presumably amplifying the inherent immunoincompetence), both colonization and dissemination increased in these animal models. Dexamethasone plus antibiotics led to an increase in the percentage of filamentous forms in the GI tract compared with antibiotics alone. In addition, introduction of C albicans strains with 2 functional copies of the INT1 gene increased the number of fungi colonizing the cecum and disseminating to extraintestinal sites.
C albicans is dimorphic, having both yeast and filamentous forms (eg, hyphae, pseudohyphae, germ tubes), and is assumed to have increased virulence in immunocompromised patients because of the filamentous forms. Filamentous forms may contribute to colonization and infection, although species that do not form filaments, such as Candida glabrata, colonize and cause invasive disease in VLBW infants.
To further examine the role of yeast and filamentous forms, researchers intravenously or orally infected antibiotic-treated and dexamethasone-treated mice using 3 strains of C albicans: (1) a wild-type strain that had both yeast cell and filamentous forms, (2) a strain with only yeast cells, and (3) a strain that was constitutively filamentous.[6] The mortality rate was significantly greater in both the wild-type (92%) and yeast-cell (56%) strains compared with the filamentous strain alone (0%). The filamentous strain had no dissemination, and cecal colonization was significantly less than that of the other 2 strains. The wild-type strain had diffuse hyphal invasion with increased tissue necrosis compared with the yeast-cell strain. The researchers speculated that the yeast forms are critically important for adherence and tissue dissemination and that hyphal formation in the tissues contributes to parenchymal destruction.
After exposure, patient factors, such as degree of prematurity, skin condition, endotracheal intubation, central vascular access, diseases (eg, necrotizing enterocolitis [NEC], focal bowel perforation [FBP]), and abdominal surgery, can contribute to fungal infection. Fungal factors that contribute to infection include the size of the inoculum and factors that favor colonization and proliferation (eg, use of broad-spectrum antibiotics, postnatal steroids, histamine type-2 [H2] antagonists, parenteral nutrition, or lipid emulsions [Malassezia species]).
No comments:
Post a Comment