I will delete all inappropriate comments or links to websites or advertising.
SO DON'T BOTHER
Tuesday 28 May 2019
Still here - solutions, solutions, solutions.
I am still here.
every problem has a solution,
pharmaceutical drugs are not it.
Me
Sunday 5 May 2019
The brain-gut axis
Focus on the gut-brain axis: Multiple sclerosis, the intestinal barrier and the microbiome
Carlos R Camara-Lemarroy
Department of Clinical Neurosciences, Cumming School of Medicine, University of Calgary, Calgary T2N 2T9, CanadaHotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary T2N 2T9, Canada. ac.secivreshtlaehatrebla@yorramel-aramac.solrac
Find articles by Carlos R Camara-Lemarroy
Luanne M Metz
Department of Clinical Neurosciences, Cumming School of Medicine, University of Calgary, Calgary T2N 2T9, CanadaHotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary T2N 2T9, Canada
Find articles by Luanne M Metz
V Wee Yong
Department of Clinical Neurosciences, Cumming School of Medicine, University of Calgary, Calgary T2N 2T9, CanadaHotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary T2N 2T9, Canada
Find articles by V Wee Yong
Carlos R Camara-Lemarroy, Department of Clinical Neurosciences, Cumming School of Medicine, University of Calgary, Calgary T2N 2T9, Canada; Hotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary T2N 2T9, Canada. ac.secivreshtlaehatrebla@yorramel-aramac.solrac;
Author contributions: Camara-Lemarroy CR conceived the study and drafted the manuscript; Metz LM and Yong VW revised and approved the final version of the article.
Supported by the Lejoie-Lake Fellowship (to Camara-Lemarroy CR) awarded by the Hotchkiss Brain Institute.
Correspondence to: Carlos R Camara-Lemarroy, MD, Academic Fellow, Postdoctoral Fellow, Department of Clinical Neurosciences, Cumming School of Medicine, University of Calgary, 1403 29 Street NW, Calgary T2N 2T9, Canada. ac.secivreshtlaehatrebla@yorramel-aramac.solrac
Telephone: +1-403-9444253 Fax: +1-403-2707162
Received 2018 Jul 5; Revised 2018 Jul 30; Accepted 2018 Aug 1.
Copyright ©The Author(s) 2018. Published by Baishideng Publishing Group Inc. All rights reserved.
This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial.
Abstract
The brain-gut axis serves as the bidirectional connection between the gut microbiome, the intestinal barrier and the immune system that might be relevant for the pathophysiology of inflammatory demyelinating diseases. People with multiple sclerosis have been shown to have an altered microbiome, increased intestinal permeability and changes in bile acid metabolism. Experimental evidence suggests that these changes can lead to profound alterations of peripheral and central nervous system immune regulation. Besides being of pathophysiological interest, the brain-gut axis could also open new avenues of therapeutic targets. Modification of the microbiome, the use of probiotics, fecal microbiota transplantation, supplementation with bile acids and intestinal barrier enhancers are all promising candidates. Hopefully, pre-clinical studies and clinical trials will soon yield significant results.
Keywords: Multiple sclerosis, Microbiome, Intestinal barrier, Bile acids, Gut-brain axis
Core tip: Many studies suggest that the brain-gut connection can contribute to our knowledge of the pathophysiology of neurological conditions. Recent evidence suggests that people with multiple sclerosis have changes in their gut microbiome, their intestinal barrier and even in the metabolism of bile acids. All of these represent relevant therapeutic targets that could feasibly be addressed by pre-clinical and clinical studies. This knowledge acquired in the bench might soon be translated to the bedside.
INTRODUCTION
Clinical and preclinical studies have shown bidirectional interactions within the brain-gut axis and the gut microbiome, the intestinal barrier and the immune system, both in health and disease. These complex interactions might be relevant for the pathophysiology of inflammatory demyelinating diseases, and in particular, multiple sclerosis, where much interest has been placed in the recent literature.
THE GUT MICROBIOME
Much interest has been placed recently on the possible role of the gut microbiome in multiple sclerosis (MS) pathophysiology. Many review articles on this subject have recently been published[1-3], perhaps more than original research articles that actually characterize the gut microbiome in patients with MS. This research is in keeping with the essential role that the gut microbiome has in regulating the development of the immune system[4]. This area of research has also been the subject of recent symposia in international MS conferences[5,6].
Much of the experimental evidence is derived from studies using the experimental autoimmune encephalomyelitis (EAE) mouse model of MS. Modifying the gut microbiota with either antibiotic cocktails or probiotics leads to EAE attenuation as well as a multitude of regulatory immune responses[7-9]. Animals bred in germ-free conditions are resistant to EAE induction and show an attenuated immunological response[10,11], an effect lost when mice are repopulated with gut commensals[11]. In recent intriguing experiments, transgenic mice prone to spontaneous brain autoimmunity developed severe disease when transplanted with fecal microbiota from MS patients, as opposed to mice that received fecal microbiota from healthy matching twins[12]. Germ-free mice receiving similar transplants also developed severe EAE, while showing altered peripheral immune responses[13].
From studies attempting to characterize the composition of the microbiome, it is clear there are some differences in people with MS compared to controls. People with relapsing-remitting MS (RRMS) have an abundance of Anaerostipes, Faecalibacterium, Pseudomonas, Mycoplasma, Haemophilus, Blautia, and Dorea and a relative decrease of Bacteroides, Prevotella, Parabacteroides and Adlercreutzia[14-16]. In pediatric MS, patients have higher levels of members of Desulfovibrionaceae and depletion in Lachnospiraceae and Ruminococcaceae[17,18]. Issues are further complicated by complex analyses at the taxa, phylum and species levels, and a myriad of microbes have been implicated. For example, studies have found a significant depletion in clostridial species[15,19], Butyricimonas[20], Roseburia[21] and an increase in Streptococcus[22], Methanobrevibacter, Akkermansia and Coprococcus[14,20].
However, there are some limitations to these studies. The methods used to analyze the microbiome have been heterogeneous, with most (but not all) studies using a variation of 16S sequencing. There are differences in sample processing, DNA extraction, choice of primers, databases and hyper-variable regions analyzed across studies. Furthermore, close to two thirds of patients with MS have gastrointestinal symptoms such as constipation, dyspepsia and other functional gastrointestinal disorders[23], and many of these have been also associated with an altered gut microbiota[24]. Studies so far have not properly accounted for these symptoms or other relevant variables such as diet. An ongoing International MS Microbiome study aims to define a “core microbiome”[3]. It might shine some light into this complicated field.
Nonetheless, there is mounting experimental evidence that the gut microbiome may play a role in MS pathophysiology and human studies suggest that patients have a different microbiome compared to controls. Of course, the true significance of the results obtained so far is unclear, considering that there has often been a failure to replicate microbiome animal studies in humans. But the next question that comes to mind is whether this can also constitute a relevant therapeutic target. Although this appears to be the case in experimental models, translation to clinical practice may prove challenging.
Modifying the microbiome through medications, possibly antibiotics, could be the simplest method, but several issues arise that question the feasibility of this approach. Targeting specific commensals might prove difficult and requires appropriate identification of these targets. The case of minocycline is an interesting example. Recently shown to delay the occurrence of a second demyelinating event in patients with a clinically isolated syndrome[25], minocycline is an antibiotic known to alter the gut microbiome[26]. Whether this is an additional mechanism of action remains unknown; it is noteworthy that the initial rationale for testing minocycline in early MS is based on its various immune-modifying properties[27]. On the other hand, there is also evidence that MS disease modifying therapies (DMTs) may alter the microbiome directly[26], and indeed, it also appears that a multitude of other medications such as antidepressants, antipsychotics and immune modulators may also do so[28]. Issues such as the generation of resistant strains are also worthy of consideration.
Probiotics are a popular option but there are various issues with their practical implementation. Probiotics do not modify the host microbiome in a robust and persistent manner, although they are purported to be able to influence gut immunity and homeostasis. Despite success in showing a benefit for probiotics in animal models[29,30], there are only a handful of clinical trials in MS. Results have been preliminary, with some modest beneficial trends in clinical variables and some biochemical markers of changes in peripheral immune function[31-33]. However, they have included very small numbers of patients and the duration of these trials have been too short to shed any light onto clinically meaningful outcomes. There are many barriers to be overcome, such as selecting the appropriate formulation, dose and study design. There is also a lesson to be learned from the multiple clinical trials in inflammatory bowel disease (IBD), where despite a wealth of available studies (although heterogeneous in design and quality), the evidence supporting their clinical use is limited to carefully selected subpopulations[34,35].
Fecal microbiota transplantation (FMT) would constitute the optimal strategy to modify the gut microbiome. It has proven to be remarkably effective in managing C. difficile colitis, and isolated case reports describe beneficial effects over MS disease course, through mechanisms that remain unclear[36,37]. A clinical trial of FMT is underway[38], but even before its completion, many questions arise. It is unclear which population should be studied and what characterizes an ideal donor, not to mention the dose, route of administration and dose scheduling (single dose vs multiple doses). Patient with C. diff colitis who undergo FMT have been previously treated with antibiotics such as vancomycin and metronidazole, and presumably, have had some of their microbiota depleted. Would patients with MS require “ablation” of their microbiome before FMT? DMTs have immune modulating properties and they may also directly alter the microbiome[26], so their possible effects on the “engraftment” cannot be underestimated.
THE INTESTINAL BARRIER
The intestinal barrier is the physical and functional zone of interaction between the gut microbiome and the organism. It is a complex multi-layered structure that includes major portions of the gut immunological system and is essential for homeostasis[26]. However, it has been comparatively ignored regarding its possible role in MS pathophysiology.
In experimental models, mice undergoing EAE show an altered intestinal barrier, with increased permeability and various gross morphological changes, as well as alterations in the expression of tight junction proteins in the intestinal mucosa[39]. The peak of intestinal barrier dysfunction mirrors the peak of EAE clinical severity and preventing intestinal barrier breakdown leads to attenuation of EAE[40]. These alterations have also been associated with several abnormal immunological responses.
Patients with MS also have an altered intestinal barrier. Almost 2 decades ago, investigators found that patients with MS had increased intestinal permeability when compared to controls, using an in vivo mannitol/lactulose ratio test[41]. Increased intestinal permeability was also found to be associated with the number of peripheral CD45RO+ B cells[41]. A more recent study confirmed this finding; up to 70% of MS patients had increased intestinal permeability[42]. It has been hypothesized that an altered intestinal barrier might lead to bacterial translocation thus allowing the passage of noxious molecules such as microbial associated molecular patterns. This could then alter peripheral immune responses or allow these molecules to enter the CNS and alter neuroimmunity[26,43].
Although the evidence linking the intestinal barrier with MS is much more limited than evidence linking MS with alterations of the gut microbiome, the question of whether it constitutes a viable therapeutic target is the same. Of course, the issue is complicated by the fact that the microbiome is essential in the regulation of intestinal barrier function, so it could be arbitrary to think of them as separate entities. An altered intestinal barrier is also a crucial aspect of the pathophysiology of IBD and celiac disease, so research from these fields has shed light on possible strategies to maintain intestinal barrier integrity.
One of the first components of the intestinal barrier is a thick mucus layer forming a protective film, enriched by secretory IgA and antimicrobial peptides and proteins. Oral supplementation with lecithin and phosphatidylcholine can adhere to the intestinal mucosa, strengthening the mucus layer and improving barrier function[44-46]. Regulators of tight junctions, such as larazotide, are under development. Larazotide is a peptide able to re-arrange tight junctions and prevent intestinal barrier dysfunction. It has been studied in patients with celiac disease with promising results[47-49]. Designing pre-clinical studies using these methods to enhance barrier function in the setting of autoimmune neurological disease should be straightforward.
Although probiotics may not be the ideal method to modify the microbiome, they have been suggested to play a significant role in modulating barrier function. E. coli strain nissle has been marketed in Europe for many years as a probiotic with beneficial effects on the intestinal barrier[50]. It has moderate evidence from randomized trials showing it may lead to remission in ulcerative colitis[51] and in the EAE mouse model of MS it reduced disease severity by maintaining intestinal barrier function[40]. VSL#3 is another probiotic mixture with putative barrier-protecting properties[52]. There is evidence of clinical effectiveness in the management of chronic pouchitis in patients with ulcerative colitis[35,53]. VSL#3 administered to a small number of MS patients leads to an anti-inflammatory peripheral immune response[33]. These two probiotic agents would be good candidates for a large, well-designed clinical trial.
Finally, we go full circle and return to FMT. It is believed that after successful modification of the microbiome, this strategy might lead to improved intestinal barrier function[54]. The gut microbiome is essential for the regulation of intestinal barrier homeostasis[55], partly through the production of short chain fatty acids (SCFA) such as butyrate, propionate and acetate. SCFAs can modulate tight junctions in the gut and modulate inflammatory responses in the intestinal mucosa[44,55]. Other interesting alternatives have also recently been described including the use of stool substitute preparations made from purified intestinal bacterial cultures derived from a single healthy donor[56]. Of course, many questions would need to be settled before clinical trials as discussed above.
BILE ACIDS
Bile acids are the main regulators of fat and fat-soluble vitamins digestion. They also significantly affect gut physiology and homeostasis. Bile acids can modulate the intestinal barrier function through complex mechanisms[57,58], and can shape the gut microbiota community. In turn, the microbiome can change bile acid metabolism[59]. Remarkably, bile acids may also modulate inflammatory signaling in the central nervous system. Ursodeoxycholic acid can inhibit the inflammatory activity of microglia in vitro[60], and tauroursodeoxycholic acid can shift microglia phenotypes towards an anti-inflammatory state through activation of the G protein-coupled bile acid receptor 1/Takeda G protein-coupled receptor 5[61]. Bile acids are also agonists of the nuclear hormone receptor farnesoid X receptor. Bile acid farnesoid X agonism led to attenuation of EAE and modulation of neuroinflammatory responses[62]. Mice fed a high-fat diet show dysregulated bile acid synthesis, gut dysbiosis and increased microglial activation[63]. Furthermore, metabolomics studies have found alterations in bile acids in patients with MS compared to healthy controls[64]. Conjugated bile acids such as ursodeoxycholic acid have been used in the management of some gastrointestinal diseases for decades. A clinical trial of bile acid supplementation in MS is underway[65].
CONCLUSION
Exciting research suggests that the brain-gut axis, once an almost esoteric concept, might yield novel therapeutic targets in neuroimmunological diseases such as MS (Figure (Figure1).1). The often-symbiotic roles of the gut microbiome, intestinal barrier and even bile acids in the regulation of neuroimmune responses is beginning to be elucidated. If future pre-clinical and clinical studies confirm the relevance of intestinal barrier dysfunction, bile acid metabolism and the gut microbiome in the pathophysiology of MS, the next step will be to translate these findings into therapeutics. Only well designed clinical trials will answer whether interventions such as FMT, probiotics or barrier protectors yield clinically meaningful results. The time is right to assess whether the gut-brain axis can be transferred from the bench to the bedside.

Alterations in intestinal homeostasis described in multiple sclerosis as therapeutic targets. Altered bile acid metabolism, altered microbiota and alterations in intestinal barrier function all lead to local and systemic alterations in immune responses that could negatively impact MS pathophysiology (grey squares). Bile acid supplementation, fecal microbiota transplantation, probiotics, antibiotics and barrier protectors are all possible therapeutic interventions (blue squares). MS: Multiple sclerosis; FMT: Fecal microbiota transplantation; PC: Paneth cells; EC: Epithelial cells; TJ: Tight junctions; L: Lymphocytes
P.2 CCSVI
While CCSVI treatment can improve venous drainage, which may further relieve hydrocephalic conditions in certain cases, it cannot improve CSF flow through the subarachnoid space of the upper cervical spine. Furthermore, increasing venous drainage of the brain and consequently decreasing CSF volume without a proportionate rise in passive CSF production could compromise brain support causing it to sink in the vault resulting in a condition similar to a pressure conus or Chiari malformation. Over drainage of the brain may thus present problems similar to spinal taps which can cause headaches due to a pressure conus condition following CSF removal. Over drainage is probably less likely in younger cases where the passive CSF pressure gradient and CSF production remains strong. Older patients, on the other hand, may have a lower CSF pressure gradient and thus a decrease in passive production of CSF due to aging of the brain and chronic craniocervical back pressure against the vertebral veins and subarachnoid space.
The flow of CSF clearly plays a role in normal pressure hydrocephalus (NPH), which has been associated with Alzheimer’s and Parkinson’s disease. It also plays a role in Chiari malformations, which cause signs and symptoms similar to MS. I discuss CSF production and flow thoroughly in my book. I will be discussing it more here in future posts as well as on my new website at: http://www.upright-health.com/.
ADVERTISING
Uprightdoc ( 100% respect) p.1
Cerebrospinal fluid (CSF) flow is called the third circulation of the brain and it is the least understood. CSF production and flow is critical to brain cushioning and protection. In terms of protection CSF is important to brain support to prevent the brain from sinking in the cranial vault. Conversely, excess CSF volume compresses the brain.
CSF comes from arterial blood that has been filtered through the blood brain barrier to the point where it is mostly water. CSF leaves the brain through the venous system. Therefore, backups in the venous drainage system affect cerebrospinal fluid (CSF) flow and drainage. Although it uses other routes as well, such as cranial and spinal nerves and the lymphatic system, most of the cerebrospinal fluid (CSF) produced by the brain eventually makes its way up to the superior sagittal sinus where it empties into the venous system.
The superior sagittal sinus, depicted in the graphic image on the right, is the largest dural sinus located at the top of the brain. The superior sagittal sinus contains arachnoid granulations that act as one way check valves for the flow of CSF from the subarachnoid space to the sinus. Click on the image for a better view. The pulsatile nature and the pressure generated by the CSF outflow through the arachnoid granulations is powerful enough to scour impressions into the roof of the cranial vault.
About sixty percent of the CSF produced in the brain ends up in the spinal cord. Eventually most of the CSF in the spinal cord makes its way back up through the subarachnoid space of the cord and into the subarchnoid space of the brain. From there it travels up to the superior sagittal sinus and arachnoid granulations to exit the brain along with venous blood.
The movement of CSF is driven by cardiovascular waves arising from the heart and blood vessels. During the contraction phase of the heart cycle (systole) pressure in the arteries of the brain increases. The increase in blood pressure drives CSF out of the brain through the upper cervical spine because as blood volume rises CSF volume must decrease. During the relaxation phase (diastole) the pressure drops and CSF enters the cranial vault through the subarachnoid space of the upper cervical spine. In addition, because the veins of the vertebral venous plexus of the spine have no valves, respiratory pressure changes are transmitted to the brain and amplify the cardiovascular waves. In brief, as pressure in the chest cavity drops during inspiration, due to the diaphram moving down and the chest wall moving out, CSF is pulled out of the cranial vault. As pressure in the chest cavity increases during exhalation CSF is driven into the cranial cavity. Thus, combined cardiorespiratory waves are important to the movement of CSF through the brain and cord.
The CSF that leaves the brain on its way down to the cord , however, must first pass through the tight neural (spinal) canal of the the upper cervical spine. Likewise, on its return trip back to the brain, it must again pass through the neural canal of the upper cervical spine. Therefore, the upper cervical spine is a critical link in the flow of CSF between the subarachnoid space of the brain and the cord. Under normal circumstances cardiorespiratory waves move CSF through the neural canal of the upper cervical spine unimpeded with good pulsatility and continue to drive it through the subarachnoid space up to the superior sagittal sinus.
Genetic design flaws, such as Chiari malformations, and acquired disorders from injuries or disease can impede the pulsatility and flow of CSF through the upper cervical spine. Restrictions in CSF flow that cause a decrease in its volume, can, in turn, cause Chiari malformations and pressure conus conditions. Furthermore, any condition that restricts CSF flow can lead to hydrocephalus-like conditions. It is therefore important to maintain the correct volume of CSF in order to provide sufficient brain support and protection, as well as to prevent hydrocephalus.
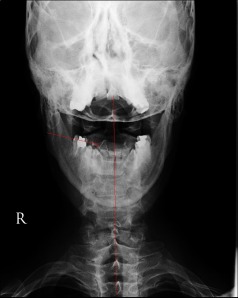
The picture above shows a fairly severe rotational misalignment of the upper cervical spine to the right. Click on the image for a better view. The dart shaped structure in the upper cervical spine is the spinous process of C2. It should be in the midline. The misalignment was caused by a motorcycle accident in which the victim landed on the right side of his head causing his head to snap to the left while simultaneously shifting and twisting his upper cervical spine to the right. Misalignments, such as the one above (due to micro or macro trauma), genetic design flaws (Chiari malformations), diseases (rheumatoid arthritis) and degenerative conditons (aging) of the upper cervical spine can affect the vertebral arteries that supply the brain, as well as the vertebral veins that drain the brain during upright posture. They can also cause deformation of the subarachnoid space and consequently, they can affect CSF flow going into and out of the brain and cord.
Subscribe to:
Posts (Atom)