An Overview of Chytridiomycosis
May 17, 2011.
- What is chytridiomycosis and what causes it? How many amphibian species are affected by it?
- Where has this pathogen been found?
- Where did Bd originate? Is it a novel pathogen?
- How is it spreading?
- Under what conditions does Bd grow?
- Is there any pattern to Bd prevalence or Bd-caused mortality?
- Does Bd kill all types of amphibians (frogs, salamanders, caecilians)? What species are most/least affected? What life stages are affected?
- How does Bd kill amphibians?
- What is the life cycle of Bd?
- What happens when Bd arrives in an area? What is the impact on the local ecosystem when Bd causes amphibian extirpation?
- How can Bd infection be detected? (swab protocol, histology, real-time PCR)
- Is there any way to cure amphibians once they are infected with Bd?
- How can people avoid spreading Bd?
- What is known about the Bd genome?
- Other links for chytridiomycosis
- References
I. What is chytridiomycosis and what causes it? How many amphibian species are affected by it?
- Chytridiomycosis is an emerging infectious disease of amphibians caused by an aquatic fungal pathogen, Batrachochytrium dendrobatidis (Bd) (Daszak et al. 2003). Amphibian die-offs in Australia led to the theory that a pathogen was devastating Australian frogs (Laurance et al. 1996); the pathogen was confirmed to be a chytrid fungus in 1998 (Berger et al. 1998) and described in 1999 (Longcore et al. 1999). Bd appears to be specific to amphibians (Berger et al. 1998; Longcore et al. 1999), and is one of only two species of chytrid fungus known to parasitize vertebrates (the other being Ichthyochytrium vulgare, which parasitizes fish; Plehn 1916; Červinka et al. 1974; Sch‰perclaus et al. 1992). Bd infection has been documented in numerous frog species, some salamander species, and a single caecilian species (Typhlonectes sp.) in captivity (Raphael and Pramuk 2007, unpublished abstract).
Bd may be responsible for the greatest disease-caused loss of biodiversity in recorded history (Skerratt et al. 2007). Over just the past 30 years, Bd has caused the catastrophic decline or extinction (in many cases within a single year) of at least 200 species of frogs, even in pristine, remote habitats (Skerratt et al. 2007). These rapid, unexplained declines have occurred around the world (e.g., Costa Rica: Crump et al. 1992, Lips 1998; Panama: Lips 1999, Lips 2003a, Lips 2003b, Lips 2006; Brazil: Heyer et al. 1988, Weygoldt 1989; Australia: Laurance et al. 1996). Recently Bd has been implicated in the unexplained disappearances of Central American salamanders as well (Rovito et al. 2009). While diseases have previously been associated with population declines and extinctions (Daszak et al. 2000), chytridiomycosis is the first emerging disease shown to cause the decline or extinction of hundreds of species not otherwise threatened (Skerratt et al. 2007). Currently over 350 amphibian species are known to have been infected by Bd (Fisher et al. 2009).
Bd infects the superficial, keratin-containing layers of amphibian skin (Berger et al. 1998). In frog tadpoles, only the mouthparts are keratinized and susceptible to Bd infection (Berger et al. 1998), leading to mouthpart depigmentation and sometimes defects (Rachowicz and Vredenburg 2004). During metamorphosis the skin of the body become increasingly keratinized and the fungal infection is then able to spread over the skin in froglets (and adults) of susceptible species (Marantelli et al. 2004; Rachowicz and Vredenburg 2004). In juveniles and adults Bd infects and encysts within skin cells particularly on the belly, digits, and pelvic "drink patch" (Berger et al. 1998). As infection proceeds the skin becomes much thicker (hyperkeratosis) and sloughs off (Berger et al. 1998). Osmotic regulation is increasingly compromised and electrolyte blood levels drop, leading to death from cardiac arrest (Voyles et al. 2009). Mortality rate and time to death post-exposure depend on a number of factors, such as pathogen dose, temperature, age and lifestage, species, and Bd strain (Berger et al. 1999, 2004; Lamirande and Nichols 2002; Woodhams et al. 2003; Rachowicz and Vredenburg 2004; Berger et al. 2005). Infection intensity appears to be a key factor; death ensues in adult frogs and salamanders once the individual has reached an infection load of about 10,000 fungal zoospores (Vredenburg et al. 2010 for frogs; Cheng et al. 2011 for salamanders).
In many frog species, Bd is highly pathogenic in the laboratory, and even low levels of initial infection can lead to death (Skerratt et al. 2007). In other frog species, such as Rana catesbeiana, Bd can infect tadpoles and adults at persistent low levels without killing them, so that these species act as carriers of the fungus (Skerratt et al. 2007; see section VI below for further discussion of anuran species that can act as carriers of Bd). In salamanders, some species appear to resist, persist with or clear Bd infections to a greater extent than do frogs (Davidson et al. 2003), suggesting they may act as reservoirs of Bd infection (e.g., the Eastern tiger salamander, Ambystoma tigrinum). Weinstein (2009) found that although infected field-collected salamanders (Batrachoseps attenuatus) had 100% mortality when brought into captivity, salamanders inoculated with Bd in captivity and housed in dry microhabitats (to mimic summer estivation) were all able to clear Bd infection. Despite these findings, Bd susceptibility appears to vary between salamander species, affecting some more strongly than others. Rovito et al. (2009) reported possible involvement of Bd in drastic, enigmatic Central American salamander declines. Subsequently, Cheng et al. (2011) developed a method for qPCR of formalin-fixed museum specimens and showed that Bd infections were present in multiple Mexican and Central American salamander species, at about the same time that declines were occurring. In addition, two neotropical salamander species (Pseudoeurycea leprosa and Bolitoglossa rufescens) were found to be quite susceptible to Bd infection in the laboratory (Cheng et al. 2011).

Photo © by Vance Vredenburg
Mountain yellow-legged frogs (Rana muscosa) killed by chytrid in August 2008.
Photo taken at Sixty Lake Basin in the Sierra Nevada mountains, California, USA.
II. Where and when has this pathogen been found?
- Bd has been found on all continents where amphibians occur; in other words, every continent except Antarctica (Fisher et al. 2009). Bd has also been linked with serious declines almost everywhere that amphibians occur (North, Central, and South America, Australia, Africa, and Europe; Van Sluys and Hero 2010). While the most dramatic mass mortalities have taken place in Australia, Central America, and North America, researchers are investigating possible Bd-related declines in other regions as well.
Amphibian Bd infections have been documented at almost the full range of elevations at which amphibians occur, from nearly sea level (in Leptodactylus fallax, on the Caribbean island of Dominica) up to 5,348 m above sea level in the Peruvian Andes (in Pleurodema marmoratum and Telmatobius marmoratus), where frogs have colonized ponds at up to 5,400 m in deglaciated areas (Seimon et al. 2006).
Worldwide distribution of Batrachochytrium dendrobatidis (Bd), the amphibian chytrid fungus 
View a larger image. Taken from Fisher et al. (2009); the image is a screenshot from
www.spatialepidemiology.net/bd-maps/
In Australia, Bd has been found in four zones, including parts of every state or territory except for the Northern Territory: (1) on the east coast, in rainforests of Queensland and New South Wales, and montane and foothill forests in Victoria (Berger et al. 1999); (2) in the southwest, extending from Perth (Berger et al. 1999); (3) around Adelaide (Berger et al. 1999); and (4) in Tasmania (Obendorf 2005; Obendorf 2006). No positive records have yet been reported from the Northern Territory (Speare and Berger 2005; Van Sluys and Hero 2010). The earliest museum specimen in each zone with signs of Bd infection dates to December 1978 for the east coast (Conondale Range, southern Queensland), October 1985 for the southwest, May 1996 for Adelaide, and 2004 for Tasmania (Obendorf 2005). At least one Australian frog species, the sharp-snouted torrent frog (Taudactylus acutirostris) is known to have been driven to extinction by Bd, with the last wild population crashing in September 1993 and the last captive animal dying from Bd in the Melbourne Zoo in 1995 (Speare and Berger 2005). Bd is also strongly suspected in the extinction of the gastric brooding frogs, Rheobatrachus vitellinus and Rheobatrachus silus, as well as the Southern day frog, Taudactylus diurnus (Laurance et al. 1996; Berger et al. 1998). Rapid amphibian declines in mainland Australia began in the late 1970s in southern Queensland, near Brisbane (Czechura and Ingram 1990) and continued northward to central eastern Queensland in the mid-1980s. In coastal Queensland the rate of advance of the pathogen has been estimated at 100 km per year (Laurance et al. 1996). In Tasmania declines also began in the late 1970s (Fearn et al. 2003).- Declines were first noticed in the late 1970s and early 1980s in the D'Aguilar, Blackall, and Conondale subcoastal mountain ranges near Brisbane, southeast Queensland (Laurance et al. 1996). Taudactylus diurnus, the Southern day frog, vanished over a period of three to four years, disappearing from the DíAguilar Range in late 1975, then from the Blackall Range in late 1978 and finally from the Conondale Range in early 1979 (Czechura and Ingram 1990). Similarly, the Southern gastric brooding frog, Rheobatrachus silus (a frog with the unusual habit of brooding its embryos in the mother's stomach and giving birth through the mouth) was not sighted in the Conondale Range after 1979 (Czechura and Ingram 1990), and was last collected in the Blackall Range in 1981 (Richards et al. 1993). Another three species in southeastern Queensland and adjacent New South Wales nearly went extinct during the same time period, with population declines of more than 90% (Litoria pearsoniana, the cascade tree frog; Mixophyes iteratus, the giant barred frog; and Mixophyes fleayi, Fleay's barred frog) (Ingram and McDonald 1993). In the mid-1980s more Australian amphibian declines were documented in central eastern Queensland, about 700 km north of Brisbane in marginally tropical rainforest. The Northern gastric brooding frog (Rheobatrachus vitellinus) was discovered in 1984, and was noted as being common (Mahony et al. 1984). The Eungella torrent frog,Taudactylus eungellensis, was also reported as common up through 1984 (McDonald 1990). Declines were seen first in lower-elevation populations (400 m above sea level) of both species in January 1985 (Winter and McDonald 1986). Despite the declines in lower-elevation populations, both species were still common at higher elevations in March 1985. However, surveys in June 1985 (just three months later) looking for higher-elevation populations of Rheobatrachus vitellinus revealed no trace of this species (McDonald 1990). Rheobatrachus vitellinus has not been recorded since March 1985; Taudactylus eungellensis has not been seen since June of 1986 (McDonald 1990). From 1989-1996, several studies reported further declines in Queensland, with two species (Litoria nyakalensis, the mountain mistfrog, and Taudactylus rheophilus, the tinkling frog) gone from their upland rainforest habitat, one species missing from most of the surveyed upland sites (Taudactylus acutirostris, the sharp-snouted torrent frog) and other species missing from most of the surveyed upland sites but persisting at lowland sites (Litoria nannotis, the waterfall frog; Litoria rheocola, the common mistfrog; and Nyctimystes dayi, the lace-eyed tree frog) (Richards et al. 1993; Trenerry et al. 1994; Hero 1996). In 1993, amphibian populations at Big Tableland (near Cookstown in north Queensland) suffered mass mortality and crashed suddenly, and this time amphibian biologists were able to collect dead frogs rather than simply noting that numbers had declined. Subsequently it was proposed by Laurance and colleagues that a pathogen, perhaps a water-borne virus, might be the cause of these amphibian extirpations, with at least fourteen species of endemic Australian rainforest frogs affected (Laurance et al. 1996). All affected species were stream-breeders. In some species, lowland populations were able to persist while high-elevation populations vanished. Declines were selective, with some species persisting and others not (Laurance et al. 1996). Because pathogens do not typically drive their hosts to extinction, this hypothesis was not initially accepted. However, examination of dead frogs from the 1993 Big Tableland die-off revealed a parasitic infection of the skin; subsequently the parasite was identified as a chytrid fungus and tests confirmed its involvement in amphibian deaths (Berger et al. 1998; Longcore et al. 1999). Retrospective examination of preserved museum specimens showed that Bd infection was not present in specimens collected in south Queensland until 1978, after which it spread north and south along the coast (Skerratt et al. 2007). In western Australia, chytridiomycosis was first detected in 1985 at a site south of Perth, and spread in all directions from the initial site of detection (Skerratt et al. 2007).
In Tasmania, the first amphibian declines occurred in the late 1970's, according to retrospective accounts from individuals living in the Launceston area, who noted a steep drop in abundance in Litoria raniformis, the southern bell frog (Fearn et al. 2003). PCR-based surveys for chytridiomycosis were first conducted in 2004, specifically testing tadpoles from a number of different species (Obendorf 2005; Obendorf and Dalton 2006). By that time declines had also been noted in an endemic Tasmanian species, the Tasmanian tree frog (Litoria burrowsae), and mass mortality events had been reported in suburban populations of juvenile Litoria ewingi, the whistling tree frog (Obendorf 2005). Bd was confirmed present in multiple habitats (high-altitude wetlands, >800 m asl, on the Tasmanian Central Plateau; peri-urban wetlands; suburban privately-owned frog ponds) (Obendorf 2005). Tadpoles of multiple species were found to be infected (Obendorf 2005). In particular, Bd infection was found most frequently in habitats that were close to major cities and towns (Obendorf and Dalton 2006), and to be much less prevalent in remote areas of the Tasmanian Wilderness World Heritage Area (TWWHA) (Pauza and Driessen 2008). The most likely method of introduction of Bd to Tasmania is thought to have been via infected frogs accidentally transported in the produce and horticultural trade, particularly in bananas (McDonald and Speare 2000; Obendorf 2005; also see section below) and potted plants (Obendorf and Dalton 2006). Within the TWWHA, Bd distribution was strongly associated with the presence of gravel roads; maintenance of these roads requires transport of water from local wetlands (used to spray the roads for dust suppression) and transport of moist soil for road repairs, both of which may move Bd zoospores or Bd-infected amphibian adults/tadpoles into new areas (Pauza and Driessen 2008). The Department of Primary Industries and Water has constructed a map of chytrid presence/absence in Tasmania as of 2009.
In North America, Bd has been found in Canada and the United States (Ouellet et al. 2005), as well as in Mexico (but not Baja), according to data stored at www.spatialepidemiology.net/bd-maps/, and Cheng et al. (2011). The earliest amphibian specimens which clearly show histological evidence of Bd date to 1961 from both Canada and the United States, and are ranid frogs. At least one ranid species, the American bullfrog (Rana catesbeiana) appears to be a carrier of Bd, harbors high diversity in Bd genotypes and may be responsible to at least some extent for transporting Bd around the world (Rosenblum et al. 2010; Garner et al. 2006; Mazzoni et al. 2003). While salamanders can be susceptible to Bd, salamander declines in the United States so far appear to be associated more with ranavirus outbreaks than with Bd, and some U. S. salamander species may be carriers of Bd (e.g., Jancovich et al. 2003). In contrast, Bd is probably involved in salamander declines in Mexico and Central America (Rovito et al. 2009; Cheng et al. 2011).
- Canadian sampling has primarily been done near the border with the United States, except for some sampling further north in British Columbia. The 1961 Bd-infected Canadian specimen is a Rana clamitans from Quebec (Ouellet et al. 2005).
- Nearly all U. S. states have tested positive for Bd. U. S. states that so far test negative for Bd are Kansas, Mississippi, and New York; states that do not yet have any Bd sample data are Kentucky, Nebraska, North Dakota, South Dakota, and Wisconsin, according to www.spatialepidemiology.net/bd-maps/The two 1961 Bd-infected United States specimens were Rana catesbeiana from central California (Padgett-Flohr and Hopkins 2009). It is thought that Bd was introduced to Santa Clara County, in northern California, in the late 1950's or early 1960's; one possible vector might have been Xenopus laevis, which is known to be a carrier of Bd and which was first shipped to the United States in 1949, perhaps to Stanford University (Padgett-Flohr and Hopkins 2009). In California, Bd has subsequently spread out geographically and is now found throughout most of central California (Padgett-Flohr and Hopkins 2009). Within the United States, Bd has been linked to crashes in Rana chiricahuensis (Bradley et al. 2002), Rana yavapaiensis (Bradley et al. 2002); Rana pipiens (Morell 1999); and Rana muscosa (Rachowicz et al. 2006).
- In Mexico, the earliest Bd-infected specimens are salamanders from the mountains of southern Mexico (Thorius pennatulus) dating to 1972 (Cheng et al. 2011). Declines were noted in Mexican salamanders during the 1970s-1980s (Parra-Olea et al. 1999; Rovito et al. 2009). Bd has also been associated with sharp declines in the frog Rana tarahumarae (Hale et al. 2005). Bd has not been found in Baja, but positive swabs have been collected in both northern Mexico and southern Mexico (see Cheng et al. 2011, and www.spatialepidemiology.net/bd-maps/).
Bd has advanced southward from Mexico through Central America 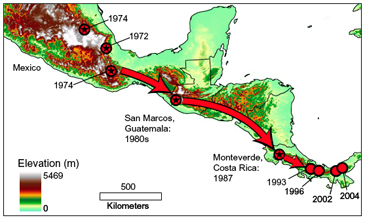 Figure from Cheng et al. 2011.
Figure from Cheng et al. 2011.
View a larger image.
In Central America, precipitous amphibian declines were first noted in the mid-1980s. Amphibian communities have collapsed sequentially along a transect advancing southward. Bd has spread in a southeastward path from North America (Mexico) through Guatemala (Cheng et al. 2011), Honduras (Cheng et al. 2011), Costa Rica (Lips et al. 2006; Cheng et al. 2011) and Panama (Lips et al. 2006) and is continuing into South America, at a rate of about 17 km/year (Lips et al. 2006).
- The best-known case occurred in 1988 when the golden toad (Bufo periglenes) vanished from its remote, pristine mountainous habitat in a Costa Rican preserve, with the last golden toad seen in 1989. At the same time in nearby Monteverde, Costa Rica, the harlequin frog (Atelopus varius) disappeared, and many other Costa Rican amphibian species either vanished or suffered catastrophic declines; by the late 1980s, 40% of amphibian species across a wide taxonomic range were gone from Monteverde. In a retrospective study of Costa Rican museum amphibian specimens, Puschendorf et al. (2006a) found that Bd was present in specimens that were part of a collection made in 1986, from both low and high elevations (it is not yet known whether Bd was present in Costa Rica before 1986).
- In Guatemala, high-elevation salamander communities collapsed beginning in the 1980s, particularly terrestrial microhabitat specialists as compared to arboreal species or microhabitat generalists (Rovito et al. 2009). It has been postulated that population density differences may explain this pattern of decline, with terrestrial species having higher density and higher Bd transmission rates (Cheng et al. 2011). For instance, two of the Guatemalan salamander species found in abundance in the 1970s (Pseudoeurycea brunnata and Pseudoeurycea goebeli) had completely disappeared from the study sites by the time of surveys in 2005, 2006, and 2007 (Rovito et al. 2009). It is difficult to pinpoint exactly when declines occurred, because civil unrest in Guatemala made survey work almost impossible during the time period from 1979-2005 (Rovito et al. 2009), but analysis of specimens collected in 1979 and prior reveals no evidence of Bd infection (Cheng et al. 2011). The earliest collected Bd-positive Guatemalan specimens (Bolitoglossa engelhardti and Bolitoglossa flaviventris) date from 1994 (Cheng et al. 2011).
- In Honduras, the first species observed to disappear was the frog Craugastor milesi, from Parque Nacional Cusuco, and other species also declined during the period from 1989 to 1995 (McCranie and Wilson 2002; Wilson and McCranie 2004), particularly in Pico Bonito National Park (Puschendorf et al. 2006b).
- In Panama, monitoring for Bd was begun in 2000, at a site (El CopÈ) ahead of the presumed epidemic wave. Neither amphibian declines nor Bd were detected during the first four years of monitoring, from 2000-2004 (Lips et al. 2006). In late September 2004 the first live Bd-infected frog was found; eleven days later the first dead Bd-positive frog was found and mortality abruptly spiked sharply with dead Bd-infected frogs found from 57% of the species and all seven frog families present at the site (Lips et al. 2006).
Bd has advanced southward through Central America (Costa Rica and Panama) In Europe, fatal outbreaks of chytridiomycosis in wild amphibians were first confirmed in PeÒalara National Park (Parque Nacional de la Cumbre, Circo y Lagunas de PeÒalara) in the Sierra del Guadarrama, central Spain, in 1997 (Bosch et al. 2001). Bd is now broadly distributed across Europe, in Spain (Garner et al. 2005), Portugal (Garner et al. 2005), Italy (Stagni et al. 2002), Switzerland (Garner et al. 2005), Great Britain (Garner et al. 2005), France (Garner et al. 2006), Germany (in imported, captive frogs; Mutschmann et al. 2000), and Luxembourg (at very low infection levels; Wood et al. 2009), with prevalence especially high in Spain and Switzerland (Garner et al. 2005). Figure from Lips et al. 2008.
Figure from Lips et al. 2008.
View a larger image.- In Spain, chytridiomycosis outbreaks occurred first in 1997, then again in 1998 and 1999 (Bosch et al. 2001). Thousands of midwife toads (Alytes obstetricans) were found dead, nearly extirpating these populations (Bosch et al. 2001). Beginning in 1999, significant chytrid-related die-offs were also seen in fire salamanders (Salamandra salamandra) in PeÒalara National Park (Bosch and MartÌnez-Solano 2006). Between the survey periods of 1982-1986 and 1999, natterjack toads (Bufo calamita) disappeared from half their original ponds, and one chytrid-infected, dying adult was collected, presumably in 1999 (MartÌnez-Solano et al. 2003). In contrast, PeÒalara National Park populations of the common toad, Bufo bufo, appear to have undergone minor chytrid-related die-offs but not mass decline, and other sympatric amphibian species have also remained stable (common tree frogs, Hyla arborea, and Iberian frogs, Rana iberica) or increased substantially in the number of ponds occupied (mountain newts, Ichthyosaura alpestris; marbled newts, Triturus marmoratus and Iberian green frogs, Rana perezi) (MartÌnez-Solano et al. 2003; Bosch and Martinez-Solano 2006). Rana perezi is usually a low-elevation species, and appears to have expanded into the high-elevation PeÒalara National Park (characterized by granitic outcrops interspersed with alpine grasslands and bogs, at 1,800-2,430 m in elevation), perhaps due to climate warming (MartÌnez-Solano et al. 2003). If R. perezi is found to be a carrier of Bd, similar to Rana catesbeiana, this may represent a situation similar to that reported by Seimon et al. (2006) in the Peruvian Andes, where Pleurodema marmoratum has colonized high-elevation ponds and possibly transported Bd to new habitat in the process (see section IV below). In Spain, Bd distribution appears to have arisen from a single recent introduction into the Pyrenees Mountains (bordering Spain and France), and either multiple introductions or a single more ancient introduction onto the Iberian Peninsula (Walker et al. 2010). The Iberian Peninsula Bd genotypes cluster with a North American clade in phylogenetic analysis, indicating that at least one introduction may have occurred from North America (Walker et al. 2010). In France, the only published report so far of Bd infections is on introduced bullfrogs, at Loir et Cher, Bordeaux, and Archachon (Garner et al. 2006). Crochet et al. (2004) found no evidence of amphibian decline in native frog populations from Languedoc, southern France, between the early 1970s and 2001. In Italy, Bd-infected introduced bullfrogs have been recorded (Garner et al. 2006). Stagni et al. (2002) also reported infected Bombina pachypus. In Great Britain, Bd-positive amphibian specimens have been recorded, though the species was not noted (Garner et al. 2005), and introduced bullfrogs have also been found to be infected (Garner et al. 2006). In Germany, numerous reports have been made of chytridiomycosis in imported and captive-bred frogs (Mutschmann 2000). One report of wild Bd-infected moor frogs (Rana arvalis) has been published (Mutschmann 2000).
- In Argentina, Bd has been found in Leptodactylus ocellatus (Herrera et al. 2005) and in Elachistocleis bicolor, in lowland northeastern Argentinian Atlantic forest (Arellano et al. 2009), and two species of Telmatobius in northwest Argentina have been reported as infected (Barrionuevo and Mangione 2006). In Brazil, Bd is widely distributed throughout the Brazilian Atlantic rainforest (Carnaval et al. 2006). Examination of Brazilian museum specimens yielded an approximate introduction date of 1981, which roughly corresponds to the first amphibian declines observed in Brazil (Carnaval et al. 2006). Introduced bullfrogs in Brazil have been recorded as Bd-positive (Garner et al. 2006) and native species have also been infected (Carnaval et al. 2005; Toledo et al. 2006). In Bolivia, Bd infection was detected for the first time in 2007, in torrent-dwelling tadpoles of the montane species Bufo quechua collected in Carrasco National Park (Barrionuevo et al. 2008). Mass mortalities have not been reported for Bolivia (Reichle 2006) but some amphibian populations are known to have declined (de la Riva 2005). In Chile, infections have now been reported in introduced, feral Xenopus laevis (SolÌs et al. 2010). Three of ten sampled sites were positive, and the rapid spread of this species in Chile may facilitate concomitant spread of Bd (SolÌs et al. 2010). In Colombia, museum specimens collected between 1968 and 2006 (672 specimens, 53 species) were examined retrospectively for the presence of Bd infections, and three species were found with positive signs (Ruiz and Rueda-Almonacid 2008). Atelopus mittermeieri, collected in 2004 in the department of Santander, was infected but did not show signs of chytridiomycosis, according to field notes. One Hyloscirtus bogotensis froglet and an adult Eleutherodactylus elegans were found dying and dead in 2005. In Ecuador, the earliest record so far dates to 1980 and is from the CaÒar area of Ecuador (Ron and Merino 2000; Lips et al. 2008). Multiple Ecuadorian species are affected (Ron and Merino 2000; La Marca et al. 2005; Bustamante et al. 2005). Bd-related amphibian declines have not yet been documented in the Amazon basin, but Bd has now reportedly been detected in Amazonian Ecuador, in water found in arboreal bromeliads (McCracken et al. 2009). In Peru, the earliest record dates to 1998, for Atelopus tricolor (Lips et al. 2008). Recent surveys have documented that high-elevation amphibian communities are collapsing in Manu National Park (Catenazzi et al. 2009). Earlier work in the Cordillera Vilconota, southern Peru, reported that high-elevation populations of Telmatobius marmoratus (collected in 2002) were reported as infected, in areas where locals had reported previous amphibian declines (Seimon et al. 2005). Bd was found in Atelopus pulcher in the Cainarachi Valley in 2003 (Lˆtters et al. 2005). Seimon et al. (2007) reported Bd infections in two anuran species (Telmatobius marmoratus, Pleurodema marmoratum), and linked increased mortality in Telmatobius marmoratus to chytridiomycosis while implicating Pleurodema marmoratum as a Bd-carrier that had recently expanded its range upwards. In Uruguay, chytridiomycosis was also recently reported in tadpoles of several species of native Uruguayan amphibians that had been collected between 2001 and 2007 (Borteiro et al. 2009). Additionally, bullfrogs introduced into Uruguay have been reported as Bd-positive (Garner et al. 2006).
In Venezuela, declines were first reported for Atelopus cruciger populations in the mid-1970s, and the earliest specimen with evidence of Bd infection is also A. cruciger, dating to 1986, from near Caracas (Guayasamin et al. 2002; Lips et al. 2008). Introduced bullfrogs have been reported to be infected (Hanselmann et al. 2004).Bd has spread as a wavefront from at least two areas in South America (Ecuador and Venezuela) In Asia, surveying has primarily been opportunistic rather than systematic, with the exception of that done in Japan by Goka et al. (2009). Bd has recently shown to be present on mainland Asia as well as in SE Asia. In China, Bd has been found in Yunnan Province, both in three of four native amphibian species surveyed and in non-native bullfrogs (Rana catesbeiana). In Korea, Bd has been detected in three of seven species of wild frogs surveyed (Yang et al. 2009). It has also been found on wild and captive salamanders in Japan, as well as wild frogs (Goka et al. 2009; see section III below for more details), captive, non-native frogs in Japan (Une et al. 2008), four species of wild frogs in Indonesia, though at very low prevalence (Kusrini et al. 2008), and wild frogs in the Philippines (reported in 2009 but not yet published). Within the Philippines a joint US-Philippines team of researchers has found Bd in at least five species of ranid frogs from relatively undisturbed forest at middle to high elevations (Mt. Palaypalay and Mt. Labo) on the island of Luzon, with at least one of the Bd-infected frog species showing a population decline. Although there is a reference in Lehtinen et al. (2008) to the presence of Bd infections in southern Taiwan, on farms of introduced bullfrogs, it remains a personal communication from L. Schloegel and those data have not yet been published. Schloegel et al. (2009) pointed out that live bullfrogs sold in U. S. markets (NY, LA, SF) were infected with Bd (and ranaviruses), and that most shopkeepers indicated the original source of the frogs was China or Taiwan (from frog farms). Initial surveys in Hong Kong during 2005-2006 (Lantau Island, Hong Kong Island, and the New Territories) did not turn up Bd in either native amphibians (0 of 4 species) or imported non-native amphibians bought in the food or pet markets, and enigmatic declines have not been observed in Hong Kong amphibian populations (Rowley et al. 2007). However, the non-native species tested did not include Rana catesbeiana, and very low numbers of specimens were tested for two of the three imported species (Hoplobatrachus rugulosus, 129 specimens; Occidozyga martensii, 1 specimen; Xenopus laevis, 7 specimens). An earlier retrospective histological study detect any signs of Bd infection in a small number of museum specimens from mainland China (Ouellet et al. 2005), nor did a survey of native Rana dybowskii in northeastern China (Wei et al. 2010). Lehtinen et al. (2008) did not detect Bd in their sample (20 individuals from a total of 12 species) from Lien Hua Chih Forestry Station, Nantou County, in central Taiwan. McLeod et al. (2008) found no evidence of Bd in Thailand amphibians. Likewise, an earlier survey of introduced bullfrogs (Rana catesbeiana) found no evidence of infection in Japanese populations (Garner et al. 2006). Figure from Lips et al. 2008.
Figure from Lips et al. 2008.
View a larger image.
In Africa, the earliest specimens showing signs of chytrid infection consist of a specimen of Xenopus fraseri from Cameroon, collected in 1933 (Soto-Azat et al. 2010) and a specimen of Xenopus laevis from South Africa, collected in 1938 (Weldon et al. 2004). So far Bd infection has been reported from 13 countries in Africa (Botswana, Cameroon, Democratic Republic of Congo, Ghana, Kenya, Lesotho, Malawi, Nigeria, South Africa, Swaziland, Tanzania, Uganda, and Zambia), with most occurrences in southern and eastern Africa; Xenopus species were reported to be infected in nine of those countries (Botswana, Cameroon, Ghana, Kenya, Malawi, South Africa, Swaziland, Tanzania, Uganda, and Zambia (see Blackburn et al. 2010 for a discussion and a list of citations for each country). To date, chytridiomycosis has been definitively implicated in the decline of only a single African amphibian species, the Kihansi spray toad (Nectophrynoides asperginis) (Weldon and du Preez 2004; Channing et al. 2006). Although Blackburn et al. (2010) reported an enigmatic die-off in a Cameroonian montane endemic, Xenopus longipes, they also found no evidence of chytrid involvement in this decline.
Bd has so far not been found to occur in Madagascar, which has a rich endemic amphibian fauna, and enigmatic declines (those with causes other than obvious habitat loss) of Malagasy amphibians have not been seen (Weldon et al. 2008; Andreone et al. 2008). However, niche modeling analysis predicts that central and eastern Madagascar habitat would be suitable for Bd (Ron 2005). Various strategies are being implemented as part of a Madagascar Amphibian Conservation Action plan to prevent Bd introduction, monitor amphibian populations for chytridiomycosis, and initiate captive-breeding programs in the event of chytrid detection (see Andreone et al. 2008).
In the Caribbean, Bd was first detected in Puerto Rico in the mid-1970s (Burrowes et al. 2008), decimating amphibian populations, particularly in the 1990s (Burrowes et al. 2004). Bd has persisted in Puerto Rican amphibians as an enzootic infection, even in direct-developing frogs that do not enter water to breed (Longo et al. 2009). On Dominica, a chytridiomycosis outbreak in 2002 devastated populations of the mountain chicken frog (Leptodactylus fallax), the largest native Caribbean amphibian species, within just a few months (McIntyre 2003). Montserrat was viewed as the last stronghold for Leptodactylus fallax, and initial surveys from 2003-2005 showed that chytrid was not present on Montserrat during those years (Garcia et al. 2007). In Cuba, a heavily infected and dying Bufo longinasus was found in October of 2006 (DÌaz et al. 2007). In February 2009 dead and dying frogs were found at several sites on Montserrat, prompting an evacuation of 40 apparently healthy Leptodactylus fallax to three different institutions for establishment of a captive breeding program (GarcÌa et al. 2009). Chytrid infections have also been detected on the island of Tobago, in three separate populations of the Bloody Bay frog, Mannophryne olmonae (Alemu I., 2008). This dendrobatid species is thought to have undergone precipitous decline in recent years but currently appears to be persisting with Bd as an enzootic infection (Alemu I., 2008). In New Zealand, both native and introduced species have been affected by chytridiomycosis. Amphibian declines were first seen in 1995, when dead specimens of a rare endemic, Leiopelma archeyi (Archey's frog), were collected on Tokeata Ridge in the Coromandel Peninsula, North Island, following a drought (Bell et al. 2004). In 1996, another Leiopelma archeyi population drop was noted, on Tapu Ridge. In 1998, the surviving frogs on Tokeata Ridge declined further (Bell et al. 2004). The first detection of Bd was made in November 1999 at Christchurch, in the introduced Australian species Litoria raniformis (Norman and Waldman 2000; Waldman et al. 2001), and the second was made in 2000 with the discovery of infected Litoria ewingii, also an introduced Australian species (Bishop 2000; ). In 2001 a single Leiopelma archeyi was found dead in near-pristine habitat at Te Moehau, Coromandel Range, North Island, infected with Bd (Bell et al. 2004). Chytridiomycosis susceptibility appears to vary both between species and between populations of the same species in different localities. During 1996-2001, when the terrestrial frog Leiopelma archeyi was experiencing declines on the Coromandel Peninsula, sympatric populations of the semi-aquatic Leiopelma hochstetteri did not decline dramatically (Bell et al. 2004). Further, a population of Leiopelma archeyi on the western part of the North Island, in Whareorino Forest, showed some individuals infected with Bd but did not crash (Bell et al. 2004).
III. Where did Bd originate? Is it a novel pathogen?
- It is not known with certainty where Bd originated. Weldon et al. (2004) examined museum specimens of Xenopus laevis, the African clawed frog, which is used in many laboratories for research on developmental biology. These researchers looked at skin samples from the webbing between the toes and found that the earliest specimen with visible signs of chytrid infection dated from 1938. This coincides approximately with the time period when African clawed frogs were being more widely shipped around the world for use in scientific research and pregnancy testing, beginning in the 1930s (for a discussion of the history of Xenopus pregnancy tests, see Gurdon and Hopwood 2000).
Morehouse et al. (2003) looked at genetic diversity in 35 strains of amphibian chytrid fungus from North America, Africa, and Australia, and found an extremely low level of sequence differences between strains. Since genetic diversity accumulates over time, the fact that there was almost no variation found implies that the strains from different continents have a very recent common ancestor and have not been evolving separately for very long. This supports the idea that Bd has been relatively recently introduced to different locations. Sequence analysis by James et al. (2009) of 59 Bd strains from around the globe also gave results consistent with clonal reproduction and a rapid, recent expansion in range. The source of such an introduction is not known, though unintentional human-mediated transport is strongly suspected (Halliday 1998; Berger et al. 1999; Daszak et al. 1999; Morgan et al. 2007).
Goka et al. (2009), in contrast, have reported diverse haplotypes of Bd present in Japan, with some strains appearing to be endemic to certain species of Japanese native amphibians. The highest diversity of haplotypes was found on the introduced bullfrog Rana catesbeiana. In general, Bd was found at low prevalence in field surveys of Japanese amphibians, with no evidence of an increase in prevalence around areas inhabited by infected bullfrogs. However, Bd was present at high prevalence in the native salamanders Andrias japonicus and Cynops ensicauda. Despite high Bd prevalence, chytridiomycosis disease symptoms have not been reported for captive-raised or wild-caught A. japonicus, suggesting that the Bd strains specific to A. japonicus have had a commensal relationship with this host salamander species for a long period. In addition, these authors report having identified signs of Bd infection in formalin-fixed, ethanol-stored museum specimens of A. japonicus collected as early as 1902. The combination of high diversity and apparent long-standing asymptomatic Bd infection of native Japanese amphibian species has led Goka et al. (2009) to postulate that Bd may have arisen in Asia.
Two hypotheses have been put forth to explain Bd's spread: first, that it is a novel pathogen, with the spread to new host species and new geographical areas mediated by humans (Berger et al. 1999; Daszak et al. 1999); or second, that it is an endemic pathogen which has become more virulent or to which amphibians have been rendered more sensitive due to environmental changes (Rachowicz et al. 2005; Pounds et al. 2006). The balance of evidence favors the novel pathogen hypothesis, as detailed below.
As Fisher et al. (2009) point out, the novel pathogen hypothesis is also supported by the facts that Bd is not universally distributed, that fronts of introduction have been identified in several places where catastrophic amphibian declines have occurred (e.g., Australia: Laurance et al. 1996, Berger et al. 1998; Central America: Berger et al. 1998, Lips et al. 2006; South America: Lips et al. 2008), and that infected amphibians which could serve as vectors for pathogen transmission have been found in both the wild (e.g., Cunningham et al. 2007; Garner et al. 2006; Walker et al. 2008) and in global commercial trade (e.g., Mazzoni et al. 2003; Fisher and Garner 2007). In addition, sequence and heterozygosity analysis of 59 Bd strains from around the world are consistent with global dispersal of a single, asexually reproducing (clonal) diploid lineage (James et al. 2009). Despite sampling of Bd genotypes from Africa, the Americas, Australia and Europe, there is as yet no clear phylogenetic signature of where Bd might have originated (James et al. 2009). PCR analysis of museum specimens from Mexico, Costa Rica, and Guatemala showed that Bd infections coincided with periods of known amphibian declines but were not detected in specimens collected prior to that period (Cheng et al. 2011). In Central and South America, once Bd moves into a new area, amphibian communities have collapsed; exposure of a naive host population seems to be sufficient without any need to invoke climate change (Lips et al. 2008; Cheng et al. 2011). Also arguing for the novel pathogen hypothesis and against the idea that climate change can potentiate Bd, Skerratt et al. (2007) note that the timing of a number of Australian amphibian declines did not appear to be associated with specific climatic events; that Australian seasonal temperature fluctuations are greater than those proposed for climate change; and that chytridiomycosis prevalence is decreasing in Queensland amphibian populations where Bd is now endemic, despite warming and drought. In addition, Vredenburg et al. (2010) did not detect Bd in intensive sampling of Sierra Nevada populations of Rana muscosa and Rana sierrae prior to die-offs, but once Bd infection was detected, both prevalence and intensity of infection increased very rapidly, and death ensued once a frog had been infected with about 10,000 zoospores. Walker et al. (2010) found that Bd is a novel pathogen that has been recently introduced and spread locally in the Pyrenees Mountains, Spain, but that either multiple introductions or a single ancient introduction may have occurred onto the Iberian Peninsula, with at least one introduction most likely from North America (based on phylogenetic analysis of genotypes). Those supporting the endemic pathogen hypothesis have noted that Bd has been present in some areas for a long time before declines occurred, in some cases decades (Japan: Goka et al. 2009; South Africa: Weldon et al. 2004; Canada: Ouellet et al. 2005); that Bd and chytridiomycosis distribution are strongly influenced by environmental variables (e.g., see Ron 2005 for niche modeling of Bd, and Hale et al. 2005 for the example of Rana tarahumarae); that there are some measurable associations between climate change and chytridiomycosis (Pounds et al. 2006; Bosch et al. 2007); and that there are also measurable associations between climate change and body condition, leading to decreased survival/fitness of female amphibians and potentiating higher susceptibility to pathogens (Reading 2007). Walker et al. (2010) found that pathogen (Bd) presence in Iberia was not dependent on environmental variables but that disease (chytridiomycosis) presence was weakly dependent on environmental variables (minimum temperatures, mean solar radiation), and had a strong association with higher altitude (>1600 m asl).
IV. How is Bd spreading?
- There is ample evidence to suggest that Bd is being spread through human actions, but so far no concrete evidence on how it moves naturally through the environment (i.e., whether wind-borne, transmitted via alternate hosts, etc.). Bd (along with other amphibian pathogens such as ranaviruses) is an unintentional beneficiary of the international amphibian trade (Fisher and Garner 2007). It is being carried via species exported globally for human consumption (mainly bullfrogs, Rana catesbeiana; see Mazzoni et al. 2003; Schloegel et al. 2009; Bai et al. 2010), the international pet trade (e.g., Une et al. 2008), and the scientific trade (Xenopus laevis, Weldon et al. 2004, Weldon 2005; Silurana tropicalis, Reed et al. 2005). It may also be spreading through the bait trade (mainly larval Eastern tiger salamanders, Ambystoma tigrinum, exported within the United States); Picco and Collins (2008) reported Bd-positive water samples from three of nine bait shops, although they noted that only one of the three Bd-positive water samples also had a corresponding positive PCR test from larval tiger salamander foot swabs.
Bd is also likely to be spreading via amphibians inadvertently translocated in produce. Obendorf (2005) concluded that such human-mediated transmission had occurred in Tasmania at least as early as 1993, based on his review of a pathology case reported by the DPIPWE (Department of Primary Industries, Parks, Water, and Environment) Animal Health Laboratories in Launceston, Tasmania. A captive colony of Litoria burrowsae, the Tasmanian tree frog, developed symptoms consistent with chytridiomycosis (lethargy, severe skin lesions, mortality) after a tree frog found in a banana box that had been imported from the Australian mainland was placed in the colony (Obendorf 2005). McDonald and Speare (2000) estimated that up to 50,000 frogs per year are accidentally carried in produce. O'Dwyer et al. (2000) estimated that at least 7,130 frogs per year are transported into New South Wales, Australia in shipments of bananas, of which at least 70% are released at the point of destination. Hardman (2001; cited in Obendorf 2005) surveyed commercial banana wholesalers and retailers in the Hobart area, Tasmania, and estimated that 28-90 frogs per year were found (and that more had likely escaped detection) in Hobart alone, just in banana boxes. Furthermore, 73% of Hardman's (2001) survey respondents stated that frogs found in produce were either kept by employees or released into surrounding urban areas, wetlands, bushland, or parklands. Yet another example of human-mediated transport of Bd comes from Tasmania. Within the Tasmanian Wilderness World Heritage Area, Bd distribution is strongly associated with the presence of gravel roads (Pauza and Driessen 2008). In particular, gravel roads are regularly sprayed down by trucks using water from local wetlands; this water then runs off into wetlands adjacent to the gravel road (Pauza and Driessen 2008). Also, moist soil is transported to use in road maintenance. All of these activities have the potential to move Bd zoospores and/or Bd-infected amphibian adults/tadpoles into new areas. (Pauza and Driessen 2008). Additionally, climate change can potentiate Bd's spread indirectly; as temperatures have warmed, many amphibian species (e.g., Bustamante et al. 2005; Raxworthy et al. 2008) as well as plant, insect, and other animal species are expanding (or shifting) their elevational range upwards (for a general review on climate change-induced elevational range expansion see Parmesan 2006). Amphibian species that can harbor chytrid infections without succumbing to disease may introduce Bd into new areas as they move upwards in elevation (Seimon et al. 2006). In the Peruvian Andes, recent deglaciation has allowed amphibian colonization of high-altitude ponds at record elevation levels; one anuran species (Pleurodema marmoratum) that has colonized these ponds tests positive for Bd but shows no signs of chytridiomycosis, while another colonizing species that tests positive for Bd (Telmatobius marmoratus) has experienced die-offs (Seimon et al. 2006).
V. Under what conditions does Bd grow?
- In culture, Bd is able to grow at temperatures from 4-25 °C, and over a wide range of pH, from 4-8 (Piotrowski et al. 2004). Growth is maximal at a pH of 6-7 in culture (Piotrowski et al. 2004). Bd pathogenicity and virulence are highest at temperatures between 12-23 °C (about 54-73 °F), with both pathogenicity and virulence dropping off significantly at temperatures above 27 °C (81 °F) (Berger et al. 1998, 2004; Longcore et al. 1999; Woodhams et al. 2003; Carey et al. 2006).
Experiments comparing Bd growth on autoclaved shed snakeskin vs. agar containing 1% keratin and 1% tryptone vs. liquid media consisting of 1% tryptone in distilled water found that the liquid media best supported Bd growth in culture (Piotrowski et al. 2004). Often TGhL broth is used, which contains tryptone, gelatin hydrolysate, and lactose in water (e.g., Retallick and Miera 2007). However, it has been suggested that repeated passaging in artificial media can, over time, lead to a loss of virulence in laboratory-kept Bd strains (Berger et al. 2005; Retallick and Miera 2007). An examination of the literature shows that Bd is rarely if ever passaged on frog skin in the laboratory; perhaps this should be changed. Preliminary results from comparing gene expression patterns of Bd grown on autoclaved frog skin vs. 1% tryptone solution revealed 57% of genes showed differential expression, while there was no difference in gene expression for Bd grown on shed snake skin vs. 1% tryptone solution (Rosenblum 2009).
Bd has been considered an aquatic member of the Chytridiomycota, since it requires water for its aquatic zoospore stage. Desiccation of monolayer cultures in 96-well plates, accomplished by removing liquid media and placing the open plates into a biohazard laminar flow hood for at least three hours, killed 100% of Bd zoospores (Johnson et al. 2003). However, some strains of Bd can survive in pre-sterilized soil that is merely damp, with a moisture content down to 10%, for as long as twelve weeks (Johnson and Speare 2005). This fungus can also survive in pre-sterilized sand and bird feathers for up to twelve weeks (Johnson and Speare 2005). Current research is focusing on whether Bd can form part of a biofilm (a microorganismal community encased in an exopolysaccharide matrix), which may aid survival of the fungus at higher temperatures (Carty 2009). Detection of Bd in water samples has proven difficult, however. At a Central American site (El CopÈ, Panama) where precipitous amphibian mortality was observed to occur coincident with Bd infection on dead animals, Bd was not detected in 1 L water samples (Lips et al. 2006). However, this may have been due to sampling error resulting from low zoospore concentration rather than to a complete lack of zoospores (Lips et al. 2009). Cossel and Lindquist (2009) suggested that samples of 4 L were necessary to detect Bd zoospores; in their 1 L water samples from Chiriqui, Panama, Bd levels were less than 1-2 zoospores per liter. When larger water samples of 4 L were tested, Cossel and Lindquist (2009) found that zoospores were present at detectable levels (5.1-7.5 zoospores per liter on average).
VI. Is there any pattern to Bd prevalence or Bd-caused mortality?
- The hardest-hit frog species have been subtropical or tropical stream-breeders at moderate to high elevations, where temperatures are cool. However, in North America and Spain, Bd has also impacted alpine species that experience temperatures down to freezing during the winter (Bosch et al. 2001; Muths et al. 2003; Scherer et al. 2005).
Bd has appeared in pristine, remote mountaintop habitats as well as more disturbed habitat. The fungus has an aquatic life stage (the zoospore) and may thus spread via waterways (but dispersal may also be possible by other means; see the paragraph on Sierra Nevada amphibians below). In Central America Bd has advanced southeast from Costa Rica, causing a wave of associated amphibian die-offs (Lips et al. 2006). The impact is catastrophic (Lips et al. 2003b). Once Bd enters an area, within four to six months more than 50% of local amphibian species are extirpated completely; even for species that survive, 80% of individuals die (Lips et al. 2003b). Stream-associated amphibian species are affected most rapidly and severely, with the pathogen then spreading into terrestrial amphibian species (Lips et al. 2006).
In the Sierra Nevada mountains (California, U.S.A.), Bd has caused high mortality of Sierra Madre yellow-legged frog (Rana muscosa) and Sierra Nevada yellow-legged frog populations (Rana sierrae). Each year Bd has advanced to new lakes within the Sixty Lake basin, extirpating frog populations. In contrast to the situation in Central America and within a single lake basin in the Sierra Nevadas, however, the pattern of Bd spread between Sierra Nevada lake basins has been upstream, implying an overland transmission (see Vredenburg et al. 2010). It is not yet known what is facilitating this apparent overland spread of Bd in the Sierra Nevada, but current research by the Vredenburg lab at San Francisco State University is focusing on the possible role of Pseudacris regilla, the Pacific treefrog, as a carrier of Bd, as proposed by Padgett-Flohr and Hopkins (2009).
Bd prevalence has been reported to be seasonal in temperate areas, with a higher incidence during cooler months and a lower incidence during warmer months. In Australia, a pattern of higher chytridiomycosis prevalence in winter months has been reported for frogs by numerous investigators (e.g., Aplin and Kirkpatrick 2000, for Western Australia; Retallick et al. 2004, McDonald et al. 2005, Woodhams and Alford 2005, for northern Queensland; Kriger and Hero 2007, for southeastern Queensland). Although tadpoles generally do not die from chytridiomycosis, seasonality in Bd infection occurred in tadpoles in Tasmania, with high prevalence and severe oral chytridiomycosis lesions observed in late winter and early spring, but lower prevalence and lower severity of lesions in tadpoles at the same sites at summer's end (Obendorf 2005). Similarly, in Quebec, Canada, lower Bd infection prevalence in summer months was reported by Ouellet et al. (2005). In the U. S., Pearl et al. (2007) reported higher Bd infection prevalence in Oregon and Washington during winter and spring field surveys. In Arizona, three native frog species were reported to have suffered Bd-related die-offs only in winter (Rana chiricahuensis, Rana yavapaiensis, Hyla arenicolor); winter temperatures were estimated at 16.9 °C for air and 12.9 °C for water, while summer temperatures averaged 30.2 °C and 25.8 °C for air and water respectively (Bradley et al. 2002). This seasonality fits with the thermal preferences of Bd in laboratory tests, where Bd has been found to grow best at 12-23 °C (about 54-73 °F), dropping off in both virulence and pathogenicity above 27 °C (81 °F). Bd-related mortality may also be influenced by microhabitat thermal conditions (Retallick et al. 2004). Retallick et al. (2004) noted that while most populations of Taudactylus eungellensis had been extirpated by Bd, a few remnant populations were persisting with chronic Bd infection. Sites where these frogs were extirpated (Doolomai Falls and Tree Fern Creek in Eungella National Park) had shaded, cool, small stream habitat and winter temperatures (maximum of 23 °C air/water) that fell within thermal optima for Bd growth. In contrast, sites where these frogs persisted despite continuing Bd infection (Rawson Creek) were warmer habitat; larger streams had greater canopy gaps and therefore sunnier habitat, plus much warmer summer temperatures of up to 37 °C (Retallick et al. 2004) and presumably warmer winter temperatures as well.
VII. Does Bd kill all types of amphibians (frogs, salamanders, caecilians)? What species are most affected and least affected? What life stages are affected?
- Chytridiomycosis appears to be affecting frogs to a greater extent than salamanders, although far less research has been conducted on salamanders, and new data indicate that neotropical salamanders may also be crashing due to chytridiomycosis (Rovito et al. 2009; Cheng et al. 2011). For frogs, species that live and/or breed in permanent water (particularly streams) at higher elevations appear to be the most susceptible. There are virtually no data available on chytrid infection in caecilians, which are mostly terrestrial burrowing animals (though some are aquatic or semi-aquatic) and are rarely seen. A single (unpublished) abstract on captive caecilians indicates that at least one species of aquatic caecilian (Typhlonectes sp.) can become infected with chytrid, and that infection was successfully cleared by increasing the water temperature for a period of time (Raphael and Pramuk 2007, unpublished).
Confirmed extinctions due to Bd include the sharp-snouted day frog (Taudactylus acutirostris; Schloegel et al. 2005), the Northern gastric brooding frog (Rheobatrachus vitellinus; Retallick et al. 2004), and the Southern gastric brooding frog (Rheobatrachus silus; Retallick et al. 2004), all in Australia. In Central America, Bd is strongly suspected in the extinction of the Costa Rican golden toad (Bufo periglenes), although this has not been confirmed. Because this species occurred only within a remote, high-elevation protected reserve and appeared each year only during a three-week breeding season, no preserved specimens of Bufo periglenes exist from the short period of precipitous decline and extinction (1988-1989). However, examination of preserved specimens of other anuran species from nearby areas of Costa Rica show that chytrid infection was present in a wide taxonomic range of specimens collected during 1986 (Puschendorf et al. 2006a). The hardest-hit taxonomic group has been the anuran genus Atelopus (stream-breeding toads found in Central and South America), which has been devastated by population declines (La Marca et al. 2005). Chytridiomycosis is thought to be a primary factor in the decline and disappearance of species in this genus (La Marca et al. 2005). Most Atelopus species are local endemics and generally restricted to very limited areas along mid- to high-elevation streams (1500-3000 m a.s.l.) (La Marca et al. 2005), although some species are found as low as sea level and others as high as permanent snow (Lˆtters 2007). Of 113 described and putative species, at least 30 species appear to be extinct, having been missing from all known localities for at least 8 years (La Marca et al. 2005). Only 52 of the surviving species have sufficient data with which to evaluate population trends; of these, 81% (42 of 52) have population sizes that have been reduced by at least half (La Marca et al. 2005). Higher-elevation species (>1000 m a.s.l.) have fared the worst, with 75% (21 of 28) having disappeared entirely (La Marca et al. 2005). Although habitat loss has occurred within the ranges of many Atelopus species, it does not appear to be a major factor in the decline of most Atelopus species; 22 species declined despite occurring in protected areas (La Marca et al. 2005). In contrast, Luger et al. (2008) found that two populations of Atelopus hoogmoedi, from Surinam and Guyana, had not declined and showed no evidence of chytrid infection. Some frog species are much less susceptible to death from chytridiomycosis and may act as carriers. A partial list of frog species that have been proposed as carriers of Bd (see below for Central American species) includes the bullfrog, Rana catesbeiana (Daszak et al. 2004); the Northern leopard frog, Rana pipiens (Woodhams et al. 2008); the African clawed frog, Xenopus laevis (Parker et al. 2002; Weldon et al. 2004), the Pacific treefrog, Pseudacris regilla (Padgett-Flohr and Hopkins 2009), the Puerto Rican coqui, Eleutherodactylus coqui (Beard and O'Neill 2005), and, in Australia, members of the Litoria lesueuri complex (L. wilcoxi and L. jungguy; Retallick et al. 2004), as well as the Tasmanian species Litoria ewingii (Obendorf 2005; Ricardo 2006) and Crinia signifera (Obendorf 2005). Bullfrogs are native to eastern North America but exported worldwide as a laboratory and food species; Northern leopard frogs are native to the United States and Canada but exported as a laboratory species; African clawed frogs are native to Africa but exported worldwide as a laboratory species; Pacific treefrogs range widely across the western U. S., Canada, and Mexico; and the coqui is an invasive species that has become well-established in Hawaii, among other places. In Australia, L. wilcoxi/L. jungguy showed a high prevalence of chytrid infection that did not differ between life stages, sites or seasons in addition, populations of this species complex did not decline at the same time that other species did (namely Taudactylus eungellensis and Rheobatrachus vitellinus). In Tasmania, Ricardo (2006) found that captive Litoria ewingii metamorphs were able to tolerate high levels of Bd infection for at least 31 days after metamorphosis. Central American frog species that may be carriers of Bd include the red-eyed tree frog (Agalychnis callidryas) the cane toad (Bufo marinus) the yellow tree frog (Dendropsophus microcephalus), the smoky jungle frog (Leptodactylus pentadactylus), the masked tree frog (Smilisca phaeota), and Rana ìpipiens sp. Eî) (Lips et al. 2006). All of the Central American species mentioned above were still present at the Panamanian site of Santa FÈ (west of El CopÈ) in high abundance with a high prevalence of Bd infection after Bd-associated declines had decimated other amphibian species there (Lips et al. 2006). In addition, theBd-resistant treefrog Plectrohyla matudai (Mexico, Guatemala) has been proposed as a possible carrier of Bd into bromeliads, thus potentiating infection of bromeliad-dwelling salamanders (Cheng et al. 2011).
Bd susceptibility also varies depending on the stage of life. In frog tadpoles, Bd infects just the mouthparts (the only part of the tadpole body containing keratin), causing damage to the mouth but not direct mortality. As tadpoles undergo metamorphosis and begin to become froglets, the mouthparts are lost. However, the external skin becomes more keratinized, and the fungus is then able to spread over the body of the young frog (Marantelli et al. 2004; Rachowicz and Vredenburg 2004). Histological examination of frog skin shows that Bd infection occurs primarily on the underside of the frog, particularly on the pelvic "drink patch" and the digits (Berger et al. 1998). Newly metamorphosed froglets appear to suffer the highest mortality of any life stage (e.g., Lamirande and Nichols 2002). Since tadpoles do not generally die from Bd infections, they may also serve as carriers of Bd; in particular, tadpole-to-tadpole transfer may serve to maintain Bd in the environment, especially for species such as Litoria ewingi that overwinter in permanent lentic environments (Obendorf 2005).
With respect to salamanders and chytridiomycosis, less research has been conducted on salamanders than on frogs. Chytridiomycosis has been implicated in recent large-scale unexplained declines in Central American salamanders (Lips et al. 2006; Rovito et al. 2009; Cheng et al. 2011). As is true for frogs, some salamander species appear to be less susceptible to death from chytridiomycosis, and thus may act as carriers of Bd (for example, the Eastern tiger salamander (Ambystoma tigrinum) (Davidson et al. 2003). Syntopic salamanders and frogs (those occurring in the same habitat) may thus serve as reciprocal pathogen reservoirs for each other (Davidson et al. 2003). Conversely, Bd-resistant frogs may transmit the pathogen to susceptible salamanders; in Central America, as has been postulated for Plectrohyla matudai; this Bd-resistant frog breeds in water but spends considerable time in bromeliads, where it may be transferring chytrid fungus to bromeliad-dwelling salamanders (Cheng et al. 2011). Terrestrial salamanders that are able to estivate in summer may be less susceptible to chytrid infection; experimentally infected Batrachoseps attenuatus died in the laboratory when held under moist conditions but were able to shed the infection when housed under dry conditions (Weinstein 2009). Bd is keratinophilic, and salamander susceptibility to Bd infection in the wild might also be influenced by the fact that most species of salamanders have larvae without keratinized mouthparts, unlike frog larvae (Pough et al. 2004). The exceptions include salamander larvae of the family Dicamptodontidae, and most of the family Ambystomatidae, which do have keratinized jaw sheaths (Altig and Ireland 1984); thus, if having keratinized mouthparts confers higher susceptibility to Bd, dicamptodontids and ambystomatids should experience a greater impact of Bd than other groups of salamanders. As of yet there are insufficient data to say whether this holds true. In contrast to salamander larvae, which are carnivorous and have true teeth (Pough et al. 2004), most types of tadpoles (frog larvae) are herbivorous and generally lack teeth, instead having keratinized mouthparts specialized for rasping (scraping) algae from substrates (McDiarmid and Altig 1999). Still another factor that may influence lower Bd prevalence in salamanders is that many salamander species have terrestrial direct development, where the eggs are laid on land and hatch directly into juvenile salamanders, bypassing an aquatic larval stage (and thus avoiding exposure to the aquatic zoospore stage). However, terrestrial direct development does not preclude chytrid infection or subsequent mortality. In a study by Cheng et al. (2011), adult Pseudoeurycea leprosa were found infected in the field and adult Bolitoglossa rufescens were experimentally infected with Bd in the laboratory; all infected salamanders died once infection intensity reached about 10,000 zoospores. Resistance to chytrid may be conferred by genetically-based immune differences as well as differences in the anti-microbial skin flora, and by whether the species is able to engage in behavioral mitigation (seeking out warmer and/or drier conditions). Weinstein's (2009) results showed that at least one species of plethodontid salamander (Batrachoseps attenuatus) had 100% mortality in the lab from Bd infection but was able to clear the infection if maintained under wamer and drier conditions that mimicked summer estivation. Richards-Zawacki (2009) focused on field-infected Atelopus zeteki in Panama and showed that higher body temperature was associated with increased survival in Bd-infected frogs surveyed in the wild.
VIII. How does Bd kill amphibians?
- Infection intensity is key. Vredenburg et al. (2010) found that once frogs (Rana muscosa) reached an infection level of about 10,000 fungal zoospores, death ensued. Salamanders (Pseudoeurycea leprosa and Bolitoglossa rufescens) also died once this infection threshold of 10,000 Bd zoospores was reached (Cheng et al. 2011). With regard to the actual mechanism of death, Bd infection of amphibian skin can kill amphibians by impairing electrolyte transport to such a degree that cardiac arrest occurs (Voyles et al. 2009). In amphibians the skin is one of the most important organs, involved in respiration, hydration, osmoregulation, and thermoregulation (Duellman and Trueb 1986). Amphibians generally have thin, permeable skin, and many species breathe at least partly through their skins; some amphibians have no lungs and must breathe completely through their skin (Duellman and Trueb 1986). Amphibians also absorb water and electrolytes through their skin; frogs do so especially via a special patch of skin on the belly called the "pelvic patch" or "drink patch", which is particularly susceptible to Bd infection (Berger et al. 1998). In frogs heavily infected with Bd, blood levels of certain electrolytes (sodium, potassium, magnesium, and chloride) are abnormally low (Voyles et al. 2007, Voyles et al. 2009). When electrolytes were experimentally restored by administration of an oral electrolyte solution to infected frogs (Litoria caerulea) near death, frogs regained the righting reflex and in some cases the ability to jump (Voyles et al. 2009). Although all frogs in the experiment continued to shed skin and ultimately died from the Bd infection, death was delayed by about 20 hours for Bd-infected frogs which had been treated with electrolytes (Voyles et al. 2009). It is not known whether the disruption of electrolyte transport is due to fungal toxins (Berger et al. 1998, Voyles et al. 2009) or results from physical damage to amphibian skin cells (Voyles et al. 2009). Bd infection also generally leads to hyperkeratosis ("thickening" of the outermost keratinized layer of the skin, which may range from 2-5 times thicker than normal (Longcore et al. 1999) up to 30 times thicker than normal (Berger et al. 1998). Heavy infection can lead to increased sloughing (shedding) of infected skin (Berger et al. 1998). Clinical signs of severe chytridiomycosis in post-metamorphic frogs (juveniles and adults) include anorexia, lethargy, abnormal posture with hind legs extended, and lack of righting reflex (Berger et al. 2005). Mortality rate and time to death after Bd exposure and infection are influenced by pathogen dose, temperature, age and lifestage, species, and Bd strain (Berger et al. 1999, 2004; Lamirande and Nichols 2002; Woodhams et al. 2003; Rachowicz and Vredenburg 2004; Berger et al. 2005).
In frog tadpoles, chytrid fungus infects just the mouthparts (the only part of the tadpole body containing keratin), causing depigmentation and sometimes damage to the mouth (Rachowicz and Vredenburg 2004; Marantelli et al. 2004). Although tadpoles are not usually directly killed by Bd, chytrid fungal infection may indirectly result in mortality or lower survival. Bd infection, particularly damage to the mouthparts may hamper feeding and thus affect growth and development. In turn, slower growth and development results in a smaller size at metamorphosis, potentially affecting survival if the young froglet does not succumb to chytridiomycosis (Parris 2004).
As tadpoles undergo metamorphosis and begin to become froglets, the mouthparts are lost. However, the external skin becomes more keratinized, and the fungal zoospores are then able to enter and encyst in skin cells over the body of the young frog, particularly on the belly and pelvic drink patch (Marantelli et al. 2004; Rachowicz and Vredenburg 2004). Newly metamorphosed froglets appear to suffer the highest mortality (e.g., Lamirande and Nichols 2002).
IX. What happens when Bd arrives in an area? What is the impact on the local ecosystem when Bd causes amphibian extirpation?
- In 1998 a group of researchers led by Dr. Karen Lips established a study area at El CopÈ, Panama, monitoring amphibians along both riparian (stream-associated) and terrestrial transects, and conducting both diurnal and nocturnal surveys. Over a six-year period from 1998 to 2004, amphibian species richness at El CopÈ increased along the riparian transects and was relatively stable along terrestrial transects. Beginning in 2000, amphibians were systematically monitored for chytridiomycosis, but none was detected. In early September 2004, amphibian richness and density abruptly began to decline along the riparian transects. On September 23, 2004, a Bd-infected frog was found. Less than two weeks later, on October 4, 2004, the first dead amphibian positive for Bd was found. Within four to six months, 50% of local amphibian species were completely extirpated and the remaining species were present at about 20% of pre-crash levels. Recolonization is estimated to require 15 years or more (Lips et al. 2008).
Once Bd arrives in an area, it spreads as a wavefront. In Central America, chytridiomycosis has advanced southward with amphibian populations sequentially collapsing (Lips et al. 2006; Cheng et al. 2011). In the northern part of South America, chytridiomycosis appears to have spread from at least two separate centers of introduction (one in Ecuador and one in Venezuela; see Lips et al. 2008), in four different waves. In eastern Australia, Bd has expanded northward and southward from southern Queensland; in western Australia, Bd appears to have spread outward from a site south of Perth (Skerratt et al. 2007). In North America, monitoring of Bd invasion and spread through three high-altitude lake basins (Milestone, Sixty Lake, and Barrett Lakes) in the Sierra Nevada mountains of California, separated by 20-50 km, has also revealed a wave-like pattern of infections and amphibian population extinctions (Vredenburg et al. 2010). Sampling (1-12x per Rana muscosa population per year in Sixty Lake Basin, 1-5x per Rana muscosa population per year in Milestone Basin, and 1x per Rana sierrae population per year in Barrett Lakes Basin) by PCR analysis of skin swabs did not detect Bd between 1996 and 2004. In June 2004, Bd was detected in Milestone Basin, where it spread to nearly all Rana muscosa populations within a single year. In August 2004, Bd was detected in Sixty Lakes Basin, and in July 2005 it was found in Barrett Lakes Basin. In these two larger basins, Bd had spread to all frog populations within 3-5 years of initial detection. Adult frog counts declined 99% in Milestone Basin (from 1,680 before Bd arrival to 22 in 2008; 9 of 13 populations extinct by 2008), 98% in Sixty Lake Basin (from 2,193 to 47; 27 of 33 populations extinct), and 92% in Barrett Lakes Basin (from 5,588 to 436; 33 of 42 populations extinct). Given the rate of spread, most if not all of the surviving populations are expected to go extinct between 2010-2013 as the remaining tadpoles metamorphose and become infected with Bd. Data from eight intensively sampled Sierra Nevada populations of Rana muscosa show that when infection was first detected, initial Bd prevalence and infection intensity were low (Vredenburg et al. 2010). Infection prevalence in these populations increased rapidly, taking less than 50 days to reach 100% infection in most populations, with the longest time course about 375 days. All but one sampled population reached 100% prevalence of Bd infection (97% prevalence in that population). Infection intensity increased exponentially, with die-offs occurring once frogs had reached infection levels of about 10,000 fungal zoospores. Prevalence and infection intensity remained high even in the last surviving frogs, swabbed the following summer (these were subadults that had metamorphosed after overwintering as tadpoles). Researchers in the NSF-funded TADS project (Tropical Amphibian Declines in Streams) have been investigating what happens to stream communities when frogs and tadpoles are no longer present. Once the frogs and tadpoles die off, algae grow, and nitrogen levels change, with cascading effects both up and down the stream food web (Connelly et al. 2008). Frog-eating snakes have gone extinct while other snakes have increased. In rural West Africa, Mohneke and Rˆdel (2009) point out that alteration of freshwater ecosystems resulting in the loss of larval anurans may have significant consequences for both humans and cattle; the loss of tadpoles impacts many aspects of freshwater ecology, with consequences potentially including an increase in malaria and a significant decrease in stream health.
X. What is the life cycle of Bd?
- The Bd life cycle consists of two distinct life stages, the infectious flagellate zoospore and the thallus, which can produce one or more sessile reproductive bodies called zoosporangia (where the zoospores develop) (Longcore et al. 1999; Berger et al. 2005). At 22 °C, the life cycle in vitro takes 4 to 5 days to complete (Berger et al. 2005). The zoospore is free-living and swims through the water with its tail-like flagellum until it contacts amphibian skin, and then enters a keratinized skin cell (within the stratum granulosum or stratum corneum of the epidermis) and encysts within the cell (Longcore et al. 1999). How the zoospore invades a skin cell (and evades potential host immune defenses) is not known, but cytoplasmic extensions have been observed projecting and retracting from Bd zoospores (Longcore et al. 1999).
Once a zoospore encysts inside a skin cell, it begins development into the second stage of the Bd life cycle, the thallus, which produces the zoosporangium (Longcore et al. 1999). The thallus may be monocentric, developing into a single zoosporangium, or colonial, where internal septa develop and each thallus segment then develops into a zoosporangium (Longcore et al. 1999). From each zoosporangium, thread-like rhizoids protrude (Longcore et al. 1999). In fungi, rhizoids function as anchorage and may also serve to release digestive enzymes and to absorb digested organic material, depending on the species of fungus. Additionally, rhizoids can serve in reproduction (as is the case for Chytriomyces hyalinus, Miller and Dylewski 1981). Within each Bd zoosporangium, multiple new zoospores begin developing and one or more plugged discharge tubes, or papillae, extend from the zoosporangium to the skin cell surface (Longcore et al. 1999). Once the zoospores are mature, the plug dissolves or decays and the zoospores are then released through the discharge tube into the surrounding water (Longcore et al. 1999). Zoospores can either swim away or reinfect the same animal.
Bd is diploid and mainly appears to reproduce mitotically (i.e., clonally), based on the extremely low level of DNA variation in strains from around the world and the existence of sites with single Bd genotypes, well as the high level of fixed heterozygosity in sites with multiple Bd genotypes (Morehouse et al. 2003; Morgan et al. 2007). Strains are clearly not self-fertilizing as high levels of homozygosity are not seen (Morgan et al. 2007). However, Morgan et al. (2007) were not able to reject the null hypothesis of recombination for two of their genotypically diverse Bd sites, and pointed out that this suggests Bd may be capable of outcrossing, albeit at a low rate compared to the clonal reproduction. Fisher et al. (2009) pointed out that an alternative interpretation might be that different populations could undergo loss of heterozygosity at different rates, resulting in the false impression that sexual reproduction was occurring. In other species of chytrid fungi, sexual reproduction typically results in thick-walled, resistant sporangia (Sparrow 1960; Miller and Dylewski 1981), though such resistant sporangia have not yet been shown to exist for Bd (Longcore et al. 1999; Berger et al. 2005). Since Bd is diploid, with both high heterozygosity and low sequence diversity, this suggests that it is likely to have resulted from a mating between parental strains that were closely related and heterothallic (Fisher et al. 2009). It has not yet been determined how Bd disperses. As previously mentioned, so far no resistant spore life stage has been found for Bd (Longcore et al. 1999; Berger et al. 2005), though other species of chytrid fungi have either resistant spores that can endure desiccation and heat, or resistant sporangia, such as for the chytrid Rhizophlyctis rosea). It has thus been assumed that Bd dispersal must occur via infected amphibians (adult or larval), zoospores in water, or some alternate host (Morgan et al. 2007). Infected amphibians can clearly serve to disperse Bd, particularly those species or life stages (tadpoles) which carry Bd but are much less susceptible to morbidity or mortality from chytridiomycosis (e.g., Parris et al. 2004, see below). It is unclear whether zoospores can serve as a dispersal stage, as they have been found to swim only a very short distance (<2 cm) in culture before encysting in amphibian host skin cells, and appear to have a short lifespan with only 5% of zoospores still swimming at 24 hrs (Piotrowski et al. 2004). However, Johnson and Speare (2003) found that zoospores could survive up to three weeks in tap water, four weeks in deionized water, and seven weeks in autoclaved lake water. Parris et al. (2004) found that zoospores could survive and infect Rana sphenocephala tadpoles after zoospores had been at least six weeks in mesocosm water without an amphibian host present. A number of Bd-associated mortality events have occurred at higher elevation sites; it has been suggested that Bd could be wind-dispersed and that perhaps droplets of water in mist, clouds or fog might transport zoospores or a resting stage of Bd (Collins and Crump 2009).
With respect to alternate (non-amphibian) hosts, many have been suggested (insects, bird feathers, Australian water dragons, etc.) but none have been confirmed (Johnson and Speare 2005; Phillott et al. 2009). It is not yet clear how Bd might exist in the environment apart from amphibian hosts. There is a single report of Bd-positive swabs from the underside of rocks at the site of a mortality event in Panama (Lips et al. 2006), but only a few swabs tested positive for Bd DNA out of the many tested from that site (John Woods, Pisces Molecular, pers. comm.). While the presence of Bd DNA under a rock is not proof that viable zoospores existed apart from amphibians (Collins and Crump 2009), current research suggests that Bd may exist in biofilms (Carty 2009). Perhaps Bd might be transported attached to leaves, twigs, or debris from infected aquatic habitat (Collins and Crump 2009). It has been suggested that the fungus may survive saprobically on keratin in nature, as it can reproduce on dead frog skin for a generation after the host has died, but it has also been observed that the frog skin is quickly overrun and Bd appears to be outcompeted by oomycete fungi and bacteria (Longcore et al. 1999).
Bd life cycle

From Rosenblum et al. (2008).
View a larger image. For additional information, see Biology of the Phylum Chytridiomycota
XI. How can Bd infection be detected?
- Bd infection is suspected when dead or dying frogs are found (though other pathogens can also cause mortality: see the AmphibiaWeb overview of amphibian diseases page). Clinical signs of severe chytridiomycosis in post-metamorphic frogs (juveniles and adults) include lack of appetite, lethargy, abnormal posture with hind legs extended, and lack of righting reflex (Berger et al. 2005); in Silurana tropicalis, dark spots and lack of a slime layer were also observed in moribund frogs (Parker et al. 2002). If tadpoles are present, the mouthparts can be examined to see if unpigmented areas are present (chytrid infection can result in loss of dark coloration in tadpole mouthparts) (Rachowicz and Vredenburg 2004). In salamanders, chytrid infection may be visible as small dark spots on the ventral surface (belly), and result in skin sloughing (Davidson et al. 2003; Cummer et al. 2005; Bovero et al. 2008; Weinstein 2009). Bd infection is usually then confirmed in two ways: first, by histology (sectioning skin and looking for the presence of chytrid zoosporangia within the skin; see Berger et al. 1998) and second, by swabbing amphibians thought to be infected and testing for the presence of chytrid DNA using real-time PCR (see Boyle et al. 2004, and more information below). Nested PCR has also been used to detect Bd presence (with the first round of PCR using fungal-specific primers and the second round using Bd-specific primers; see Annis et al. 2004), but quantitative real-time PCR (also known as qPCR) is preferable as it allows an estimate of infection load. Other less commonly used ways of detecting Bd include immunohistochemistry (see Berger et al. 2002, Van Ells et al. 2003, Olsen et al. 2004) and electron microscopy (see Berger et al. 2002). Until very recently, only live specimens (or dead specimens that had not yet been fixed in preservative) could be assayed by qPCR, while museum specimens had to be assayed using histological methods. Soto-Azat et al. (2009) were able to use qPCR to detect Bd infection in ethanol-fixed amphibian specimens (stored in EtOH for 2-4 years prior to testing), but not in formalin-fixed specimens. However, Walker et al. (2008) were able to detect Bd DNA by qPCR assay of some of their formalin-fixed specimens, using a Qiagen DNeasy Blood and Tissue kit to clean up DNA prior to amplification. Subsequently, Cheng et al. (2011) have refined this method and succeeded in detecting Bd infections in a high percentage of their sample specimens identified as Bd-positive through histology; their data showed that Bd was not detectable in specimens from Costa Rica, Guatemala, and Mexico collected in years before known amphibian declines, but coincided approximately with decline timing. A new rapid protocol has also been developed to distinguish viable, motile Batrachochytrium dendrobatidis zoospores from dead ones, using a two-color fluorescence assay comprised of the fluorescent stains SYBR 14 and propidium iodide (Stockwell et al. 2010). SYBR 14 passes through intact cell membranes and fluoresces green when it binds to nucleic acids. Propidium iodide passes only through compromised cell membranes (of dead/dying cells) and fluoresces red when it binds to nucleic acids. Thus live, motile zoospores stain green while dead or dying zoospores stain red.
- Detection of Bd infection by histology
- Detection of Bd infection in live specimens with real-time PCR
a. How is the animal tested to see whether it carries chytrid fungus? A sample is collected by swabbing the underside of the adult or juvenile animal (see the AmphibiaWeb video tutorial on swabbing frogs). For larval amphibians, the tadpole mouthparts should be swabbed (see Retallick et al. 2006 for a protocol on tadpole mouthpart swabbing). The swabbing protocol for adults and juveniles involves wearing gloves and carefully swabbing the underside of the animal (particularly the "drink patch" on the belly, the underside of the thighs, and the underside of the toe webbing) 30 times with what is essentially a sterile, smaller version of a Q-tip (see swabbing protocol for ordering information). If chytrid zoospores are present, the zoospores will adhere to the Q-tip. The Q-tip is then placed into a sterile, labeled, sealed tube until the DNA can be extracted and analysis can be done by real-time PCR.
Histology of a skin section from a White's treefrog (Litoria caerulea), showing heavy chytrid infection. | Scanning electron microscopy of toe skin surface from Litoria lesueuri, showing heavy chytrid infection. | |
 |  |  |
| Figure legend: I: immature stage of zoosporangium. D: mature zoosporangium containing zoospores, where discharge papillum is visible. Arrow: empty zoosporangium, after zoospores have discharged. E: epidermis. Figure from Berger et al. (1999). | A single plugged discharge papillum is visible at the surface of each epithelial cell (see arrowheads). The plug will dissolve when the zoospores are mature, enabling discharge. Figure from Berger et al. (2005) |
b. What is real-time PCR? How does real-time PCR allow estimation of infection load?
Once DNA has been extracted from the swabs, we can assay the presence and quantity of Bd zoospores collected from amphibian skin swabs, using a technique called real-time PCR (also known as qPCR). The real-time PCR assay compares the amount of Bd DNA in the sample to a universal set of standards, which contain known quantities of Bd DNA. Below you can see output from one of our sample runs. Any samples that cross the green horizontal line are positive for Bd. Samples that cross the line earlier in the amplification process (further to the left, in the graph below) have more Bd DNA, while those that cross the line later have less Bd DNA.

Real-time PCR: an example of assay results from the Vredenburg lab at SFSU.
XII. Is there any way to cure frogs and other amphibians once they are infected with chytrid?
- There is no cure yet for wild amphibian populations infected by Bd (although some species are much less susceptible than others). Most of the treatments tried on captive animals (increasing temperature, increasing salinity, various antifungal chemicals, the antibiotic chloramphenicol, and bioaugmentation) would be impossible or impractical to carry out in the wild. Bioaugmentation to boost innate immune defenses with naturally occurring amphibian cutaneous antifungal bacteria is the one treatment which so far shows some promise. Several species of amphibian skin-dwelling bacteria have now been isolated which inhibit Bd (Harris et al. 2006, Woodhams et al. 2007). Initial trials of bioaugmentation with anti-Bd skin bacteria have successfully been carried out in captivity on Rana muscosa, the Sierra Nevada yellow-legged frog (Harris et al. 2009a) and Plethodon cinereus, the Eastern red-backed salamander (Harris et al. 2009b). The hope is that a number of wild individuals could be brought into captivity and treated to sustainably reduce susceptibility to Bd, enabling survival of enough individuals to maintain a population in a Bd-infected area. Before using such a treatment in the wild, the potential effect on other species in the local ecosystem must also be carefully considered, given disastrous effects of previous attempts to use biological control in other systems (e.g., the disastrous effect of importing cane toads (Bufo marinus) into Australia to control insect crop pests). However, there is precedent for using bacterial augmentation in agricultural contexts to control disease without negatively affecting other, non-target species (Berg et al. 2007; Scherwinski et al. 2008, cited in Harris et al. 2009), and the strategy for amphibians has been to use only bacteria that already occur on amphibian skin (Harris et al. 2009a).
Rana muscosa, the Sierra Nevada yellow-legged frog, is susceptible to Bd, but individual populations show different levels of susceptibility. While most Rana muscosa populations have suffered near-total mortality from chytridiomycosis in the wild, a few tiny populations of Rana muscosa have persisted despite the presence of Bd (Rachowicz et al. 2006). Rana muscosa populations that persist with Bd have a higher percentage of frogs with detectable cutaneous antifungal bacteria (Woodhams et al. 2007). To test whether bioaugmentation could affect Bd-induced morbidity and mortality, juvenile Rana muscosa (the life stage most susceptible to Bd) were raised in captivity from field-collected eggs (Harris et al. 2009a). Prior to any treatment, all frogs in the experiment were found to have the antifungal bacterium Janthinobacterium lividum on their skins (Harris et al. 2009a). This bacterium produces the antifungal and anti-Bd metabolite violacein, but only when present at high densities (Brucker et al. 2008). Bioaugmentation with J. lividum resulted in significant differences on all measures of morbidity and mortality between treated and untreated frogs exposed to Bd (Harris et al. 2009a). Only frogs treated by bathing in a solution containing the bacterial species J. lividum had violacein on their skin; bioaugmentation-treated frogs all grew, gained weight, and were not infected when exposed to Bd, whereas all untreated frogs exposed to Bd were infected (increasingly so over time), failed to grow, lost weight, and all but one had died by the end of the experiment at 139 days (Harris et al. 2009a). It is not yet known what the maximum time is for protection from Bd by bioaugmentation; violacein was found on treated frog skin 20 weeks after bacterial inoculation (Harris et al. 2009a) but tests at longer intervals have not yet been published. In captivity different treatments for Bd have been tried with varying degrees of success: increasing temperature, increasing salinity, various antifungal chemicals, the antibiotic chloramphenicol, and bioaugmentation (by coating amphibian skin) with certain species of bacteria that have antifungal properties (see Garner et al. 2009 for some references on these different treatments, and Young et al. 2007). No treatment has yet been consistently effective across species, but thermal treatment is currently regarded as useful for captive amphibians (Young et al. 2007). Bioaugmentation with naturally occurring anti-Bd amphibian skin bacteria also holds promise (Harris et al. 2009a, 2009b). Young et al. (2007) strongly recommend quarantining newly acquired frogs in individual containers for at least 2 months, with regular examination for signs of disease during quarantine and necropsy for any animal that dies. Skin swabs should be collected on arrival and 7 weeks post-arrival, for PCR assay to test for the presence of Bd infection (Young et al. 2007).
Thermal treatment may prove to be the safest and most effective way to treat captive amphibians and their enclosures (Woodhams et al. 2003). Frogs (e.g., Woodhams et al. 2003, Retallick and Miera 2007; Andre et al. 2008), salamanders (e.g., Weinstein 2009), and caecilians (Raphael and Pramuk 2007, unpublished) have been reported to clear Bd infections when kept at higher temperatures for a time. When experimentally infected Litoria chloris enclosure temperatures were raised to 37 °C for 8 h on two successive days (also allowing 45 min for warming and 45 min for cooling on each day), the frogs were able to completely clear chytrid infections (Woodhams et al. 2003). Similarly, experimentally infected Pseudacris triseriata were able to get rid of Bd after being held at 32 °C for five days (Retallick and Miera 2007). However, it has also been reported that this treatment does not work in all species (Marantelli, pers. comm., cited in Young et al. 2007). Bd can be killed by heating to 37 °C for 4 hours (Johnson and Speare 2003; Johnson et al. 2003). Thus Young et al. (2007) recommend holding heat-tolerant amphibian species at the highest temperature they can tolerate. Itroconazole (an antifungal chemical) has successfully been used to treat Bd-infected amphibians, both by bathing amphibians in a shallow bath (Forz·n et al. 2008; Garner et al. 2009), and orally (Young et al. 2007). However, more research is needed on whether itroconazole has negative effects on amphibians, particularly tadpoles (Garner et al. 2009). Concomitant treatment with itranaconazole (bath of 0.01% itranaconazole, 10 min. daily, 14 days) and fluconazole (bath of 0.01% fluconazole, 24 h every other day, 10 treatments total) has been used as well (Une et al. 2008). Treatment of Bd-infected Silurana tropicalis (a hardy species used in laboratory research) was successful using a commercial formalin (25 p.p.m.) and malachite green (0.1 mg/L) solution at a dilution of 0.007 mL/L of tank water for 24 hours, repeated every other day for a total of four treatments (Parker et al. 2002). However, malachite green is not recommended for threatened species, as it can cause developmental deformities (Young et al. 2007). Cages can be cleaned with 10% bleach and autoclaved twice a week (Harris et al. 2009a). Water, soil, or habitat material in contact with amphibians can be disinfected by heating to a temperature over 47 °C for 30 min (Johnson and Speare 2003; Johnson et al. 2003). Water used in amphibian enclosures should be disinfected before disposal; heating to 37 °C for 4 hours, 30 minutes at 47 °C, or 60 °C for 5+ minutes will kill Bd (Young et al. 2007).
XIII. How can a person avoid spreading Bd?
 Boots and field equipment should be cleaned with 10% bleach before moving between sites.
Boots and field equipment should be cleaned with 10% bleach before moving between sites.
Cleaning boots and field equipment (such as dipnets) with a 10% Clorox (bleach) solution in water is one of the most common (and inexpensive) ways to prevent inadvertent transportation of chytrid by humans. Other chemicals have also been used to disinfect footwear and equipment (list), and a combination of heat and drying appears effective for field clothing (Young et al. 2007). Disposable gloves (changed between specimens) should be used to handle wild frogs, with captured frogs then isolated individually in plastic bags (Young et al. 2007).
The highest risk of chytrid spread is likely through anthropogenic-mediated transport of frogs and tadpoles, whether intentional or unintentional (Obendorf 2005). The two most common ways of spreading chytrid to new sites may be the use of amphibians as fishing bait and the collection and release of frogs and tadpoles as pets (Obendorf 2005). Amphibians should not be used as bait or released back into the wild once they have been taken into captivity. All newly acquired captive amphibians should be placed in quarantine initially. See previous section (XII. Is there any way to cure frogs and other amphibians once they are infected with chytrid?) for details on how to treat individuals and cages. Note that cage water and soil substrates should also be regarded as contaminated and should not be dumped into the environment. In 2008, the World Organization for Animal Health (OIE, or Office International des Epizooties), a consortium of 174 member countries and territories, placed Bd and ranaviruses on its list of notifiable diseases (Schloegel et al. 2009). The OIE's decision means that all 172 member countries must report on the status of Bd and ranaviruses within their borders every six months. No controls have yet been imposed to prevent trade of infected frogs, but the OIE's new policy is seen as a first, important step in this direction. Initial evaluations of the OIE's new policy are positive (Schloegel et al. 2010).
XIV. What is known about the Bd genome?
The Batrachochytrium dendrobatidis genome has been sequenced. Two strains of Bd have been sequenced: JEL423, taken from Phyllomedusa lemur in Panama, and JAM81, taken from Rana muscosa in California, U. S. A. Bd genome size is estimated to be 23.7 Mb for strain JEL423 and 24.3 Mb for strain JAM81, encompassing about 9000 genes (Rosenblum et al. 2008). It is currently the only genome within the Phylum Chytridiomycota to have been analyzed.
The Rosenblum lab has also generated gene expression profiles for different life stages of Bd, uncovering candidate genes that may be involved in growth, infection, and pathogenicity (Rosenblum et al. 2008).
XV. Other links for chytridiomycosis and chytrids:
- RSS feed of current scientific literature on Bd and chytridiomycosis, via PubMed, from www.spatialepidemiology.net
Information on the chytrid genome sequence
Taxonomy: Chytrid Fungi Online website
XVI. References:
Alemu I., J. B., Cazabon, M. N. E., Dempewolf, L., Hailey, A., Lehtinen, R. M., Mannette, R. P., Naranjit, K. T., and Roach, A. C. J. 2008. Presence of the chytrid fungus Batrachochytrium dendrobatidis in populations of the critically endangered frog Mannophryne olmonae in Tobago, West Indies. EcoHealth 5: 34-39.
Altig, R., and Ireland, P. H. 1984. A key to salamander larvae and larviform adults of the United States and Canada. Herpetologica 40: 212-218.
Andre, S. E., Parker, J., and Briggs, C. J. 2008. Effect of temperature on host response to Batrachochytrium dendrobatidis infection in the Mountain Yellow-legged Frog (Rana muscosa). Journal of Wildlife Diseases 44: 716-720.
Andreone, F., Carpenter, A. I., Cox, N., du Preez, L., Freeman, K., Furrer, S., Garcia, G., Glaw, F., Glos, J., Knox, D., Kˆhler, J., Mendelson, J. R. III, Mercurio, V., Mittermeier, R. A., Moore, R. D., Rabibisoa, N. H. C., Randriamahazo, H., Randrianasolo, H., Raminosoa, N. R., Ramilijaona, O. R., Raxworthy, C. J., Vallan, D., Vences, M., Vieites, D. R., and Weldon, C. 2008. The challenge of conserving amphibian megadiversity in Madagascar. PLOS Biology 6: e118. Available online.
Aplin, K., and Kirkpatrick, P. 2000. Chytridiomycosis in southwest Australia: historical sampling documents the date of introduction, rates of spread and seasonal epidemiology, and sheds new light on chytrid ecology. In: Getting the Jump! On Amphibian Disease: Conference and Workshop Compendium. Cairns, August 2000.
Arellano, M. L., Ferraro, D. P., Steclow, M. M., and Lavilla, E. O. 2009. Infection by the chytrid fungus Batrachochytrium dendrobatidis in the yellow belly frog (Elachistocleis bicolor) from Argentina. The Herpetological Journal 19: 227-220.
Bai, C., Garner, T. W. J., and Li, Y. (2010). First evidence of Batrachochytrium dendrobatidis in China: discovery of chytridiomycosis in introduced American bullfrogs and native amphibians in the Yunnan Province, China. EcoHealth, published online 06 April 2010. DOI: 10.1007/s10393-010-0307-0.
Banks, C., and McCracken, H. 2002. Captive management and pathology of sharp snouted dayfrogs, Taudactylus acutirostris, at Melbourne and Taronga zoos. Pp. 94-102 in Frogs in the Community. Proceedings of the Brisbane Symposium, edited by A. E. O. Natrass. Queensland Frog Society, East Brisbane.
Barrionuevo, J. S., Aguayo, R., and Lavilla, E. O. 2008. First record of chytridiomycosis in Bolivia (Rhinella quechua; Anura: Bufonidae). Diseases of Aquatic Organisms 82: 161-163.
Barrionuevo, S., and Mangione, S. 2006. Chytridiomycosis in two species of Telmatobius (Anura: Leptodactylidae) from Argentina. Diseases of Aquatic Organisms 73: 171-174.
Beard, K. H., and O'Neill, E. M. 2005. Infection of an invasive frog Eleutherodactylus coqui by the chytrid fungus Batrachochytrium dendrobatidis in Hawaii. Biological Conservation 126: 591-595.
Bell, B. D., Carver, S., Mitchell, N. J., and Pledger, S. 2004. The recent decline of a New Zealand endemic: how and why did populations of Archey's frog Leiopelma archeyi crash over 1996ñ2001? Biological Conservation 120: 189-199.
Berg, G., Grosch, R., and Scherwinski, K. 2007. Risikofolgeabsch‰tzung f¸r den Einsatz mikrobieller Antagonisten: Gibt es Effekte auf Nichtzielorganismen? Gesunde Pflanzen 59: 107ñ117.
Berger, L., Hyatt, A. D., Olsen, V., Hengstberger, S. G., Marantelli, G., Humphreys, K., and Longcore, J. E. 2002. Production of polyclonal antibodies to Batrachochytrium dendrobatidis and their use in an immunoperoxidase test for chytridiomycosis in amphibians. Diseases of Aquatic Organisms 48: 213-220.
Berger, L., Marantelli, G., Skerratt, L. F., and Speare, R. 2005. Virulence of the amphibian chytrid fungus Batrachochytrium dendrobatidis varies with the strain. Diseases of Aquatic Organisms 68: 47-50.
Berger, L., Speare, R., Daszak, P., Green, D. E., Cunningham, A. A., Goggin, C. L., Slocombe, R., Ragan, M. A., Hyatt, A. D., McDonald, K. R., Hines, H. B., Lips, K. R., Marantelli, G., and Parkes, H. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proceedings of the National Academy of Sciences, USA. 95: 9031-9036.
Berger, L., Speare, R., Hines, H., Marantelli, G., et al. 2004. Effect of season and temperature on mortality in amphibians due to chytridiomycosis. Australian Veterinary Journal 82: 31-36.
Berger, L., Speare, R., and Hyatt, A. 1999a. Chytrid fungi and amphibian declines: overview, implications, and future directions. In Campbell, A. (ed.) Declines and Disappearances of Australian Frogs. Environment Australia, Canberra, pp. 23-33.
Berger, L., Speare, R., and Kent, A. 1999b. Diagnosis of chytridiomycosis of amphibians by histological examination. Zoos Print Journal 15: 184-190. Available online.
Bosch, J., and MartÌnez-Solano, I. 2006. Chytrid fungus infection related to unusual mortalities of Salamandra salamandra and Bufo bufo in the PeÒalara Natural Park (Central Spain). Oryx 40: 84-89.
Bosch, J., MartÌnez-Solano, I., and GarcÌa-Paris, M. 2001. Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricans) in protected areas of central Spain. Biological Conservation 97(3): 331-337.
Bosch, J., Carrascal, L. M., Duran, L., Walker, S., and Fisher, M. C. 2007. Climate change and chytridiomycosis in a montane area of Central Spain: Is there a link? Proceedings of the Royal Society of London, Series B 274: 253-260.
Borteiro, C., Cruz, J. C., Kolenc, F., and Aramburu, A. 2009. Chytridiomycosis in frogs from Uruguay. Diseases of Aquatic Organisms 84: 159-162. Bovero, S., Sotgiu, G., Angelini, C., Doglio, S., Gazzaniga, E., and Cunningham, A. A. 2008. Detection of chytridiomycosis caused by Batrachochytrium dendrobatidis in the endangered Sardinian newt (Euproctus platycephalus) in southern Sardinia, Italy. Journal of Wildlife Diseases 44: 712-715.
Bradley, G. A., Rosen, P. C., Sredl, M. J., Jones T. R., and Longcore, J. E. 2002. Chytridiomycosis in native Arizona frogs. Journal of Wildlife Diseases 38: 206-212.
Briggs, C. J., Knapp, R. A., and Vredenburg, V. T. 2010. Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proceedings of the National Academy of Sciences 107: 9695-9700. Available online.
Brucker, R. M., Harris, R. N., Schwantes, C. R., Gallaher, T. N., Flaherty, D. C., Lam, B. A., and Minbiole, K. P. C. 2008. Amphibian chemical defense: antifungal metabolites of the microsymbiont Janthinobacterium lividum on the salamander Plethodon cinereus. Journal of Chemical Ecology 34: 1422ñ1429.
Burrowes, P. A., Joglar, R. I., and Green, D. E. 2004. Potential causes for amphibian declines in Puerto Rico. Herpetologica 60: 141-154.
Burrowes, P. A., Longo, A. V., Joglar, R. L., and Cunningham, A. A. 2008. Geographic distribution of Batrachochytrium dendrobatidis in Puerto Rico. Herpetological Review 39: 321ñ324.
Bustamante, M., Ron, S., and Coloma, L. 2005. Cambios en la diversidad en siete comunidades de anuros en los Andes de Ecuador. Biotropica 37: 180-189.
Carey, C., Bruzgul, J. E., Livo, L. J., Walling, M. L., Kuehl, K. A., Dixon, B. F., Pessier, A. P., Alford, R. A., and Rogers, K. B. 2006. Experimental exposures of boreal toads (Bufo boreas) to a pathogenic chytrid fungus (Batrachochytrium dendrobatidis). EcoHealth 3: 5-21.
Carnaval, A. C. O. Q., Toledo, L. F., Haddad, C. F. B., and Britto, F. B. 2005. Chytrid fungus infects high-altitude stream-dwelling Hylodes magalhaesi (Leptodactylidae) in the Brazilian Atlantic rainforest. FrogLog 70: 3-4.
Carnaval, A. C. O. Q., Puschendorf, R., Peixoto, O. L., Verdade, V. K., and Rodrigues, M. T. 2006. Amphibian chytrid fungus broadly distributed in the Brazilian Atlantic rain forest. EcoHealth 3: 41-48.
Carty, N. 2009. Strategies for Batrachochytrium dendrobatidis survival and pathogenesis, Part 2. Talk presented at the IRCEB Annual Meeting on Emerging Wildlife Diseases: Threats to Amphibian Biodiversity. November 13-14, 2009, Arizona State University.
Catenazzi, A., Lehr, E., Rodriguez, L. O., and Vredenburg, V. T. 2011. Batrachochytrium dendrobatidis and the collapse of anuran species richness and abundance in the upper Manu National Park, southeastern Peru. Conservation Biology 25: 382-391.
Channing, A. , Finlow-Bates, K. S. , Haarklau, S. E. and Hawkes, P. G. 2006. The biology and recent history of the Critically Endangered Kihansi Spray Toad in Tanzania. Journal of East African Natural History 95: 117-138.
Cheng, T. L., Rovito, S. M., Wake, D. B., and Vredenburg, V. T. 2011. Coincident mass extirpation of neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis. Proceedings of the National Academy of Sciences USA. Published online before print May 4, 2011, doi: 10.1073/pnas.1105538108.
Connelly, S., Pringle, C. M., Bixby, R. J., Brenes, R., Whiles, M. R., Lips, K. R. Kilham, S., and Huryn, A. D. 2008. Changes in stream primary producer communities resulting from large-scale catastrophic amphibian declines: can small-scale experiments predict effects of tadpole loss? Ecosystems 11: 1262-1276.
Cossel, J. O. Jr., and Lindquist, E. D. 2009. Batrachochytrium dendrobatidis in arboreal and lotic water sources in Panama. Herpetological Review 40: 45.
Crochet, P.-A., Chaline, O., Cheylan, M., and Guillaume, C. P. 2004. No evidence of general decline in an amphibian community of Southern France. Biological Conservation 119:297-304.
Crump, M. L. 2000. In Search of the Golden Frog. The University of Chicago Press, Chicago.
Crump, M. L., Hensley, F. R., and Clark, K. L. 1992. Apparent decline of the golden toad: underground or extinct? Copeia 1992: 413ñ420.
Cummer, M. R., Green, D. E., and O'Neill, E. M. 2005. Aquatic chytrid pathogen detected in terrestrial plethodontid salamander. Herpetological Review 36: 248-249.
Cunningham, A. A., Garner, T. W. J., Aguilar-Sanchez, V., Banks, B., Foster, J., Sainsbury, A. W., Perkins, M., Walker, S. F., Hyatt, A. D., and Fisher, M. C. 2005. Emergence of amphibian chytridiomycosis in Britain. The Veterinary Record 157: 386.
Czechura, G.V. and Ingram, G. 1990. Taudactylus diurnus and the case of the disappearing frogs. Memoirs of the Queensland Museum 29(2): 361-365. Červinka, S., VÌtovec, J., Lom, J., Hoöka, J., and Kubů, F. 1974. Dermocystidiosis - a gill diseases of the carp due to Dermocystidium cyprini n.sp. Journal of Fish Biology 6: 689-699.
Daszak, P., Berger, L., Cunningham, A. A., Hyatt, A. D., Green, D. E., and Speare, R. 1999. Emerging infectious disease and amphibian population declines. Emerging Infectious Diseases 5: 735-748.
Daszak, P., Cunningham, A. A., and Hyatt, A. D. 2000. Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science 287: 443-449.
Daszak, P., Berger, L., Cunningham, A. A., Longcore, J. E., Brown, C. C., and Porter, D. 2004. Experimental evidence that the bullfrog (Rana catesbeiana) is a potential carrier of chytridiomycosis, an emerging fungal disease of amphibians. Herpetological Journal 14: 201-207.
Davidson, E. W., Parris, M., Collins, J. P., Longcore, J. E., Pessier, A. P., and Brunner, J. 2003. Pathogenicity and transmission of chytridiomycosis in tiger salamanders (Ambystoma tigrinum). Copeia 2003: 601-607.
De la Riva, I. 2005. Bolivian frogs of the genus Telmatobius (Anura: Leptodactylidae): synopsis, taxonomic comments,and description of a new species. Monographs in Herpetology 7: 65ñ101.
DÌaz, L. M., C·diz, A., Chong, A., and Silva, A. 2007. First report of chytridiomycosis in a dying toad (Anura: Bufonidae) from Cuba: a new conservation challenge for the island. EcoHealth 4: 172-175.
Duellman, W. E. and Trueb, L. 1986. Biology of Amphibians. McGraw-Hill, New York.
Fearn, S., Visoiu, M., and Mollison, R. 2003. A proposed management plan for the flora and terrestrial fauna of the Tamar Island Wetlands Reserve with particular reference to the threatened southern bell frog (Litoria raniformis) and its decline in the Launceston area. Report prepared for Wetland Care Australia, May 2003.
Fisher, M. P., and Garner, T. W. 2007. The relationship between the introduction of Batrachochytrium dendrobatidis, the international trade in amphibians and introduced amphibian species. Fungal Biology Review 21: 2ñ9.
Fisher, M. P., Garner, T. W. J., and Walker, S. F. 2009. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annual Review of Microbiology 63: 291ñ310.
Forz·n, M. J., Gunn, H., and Scott, P. 2008. Chytridiomycosis in an aquarium collection of frogs: diagnosis, treatment, and control. Journal of Zoo and Wildlife Medicine 39: 406-411.
GarcÌa, G., Cunningham, A. A., Horton, D. L., Garner, T. W. J., Hyatt, A., Hengstberger, S., Lopez, J., Ogrodowczyk, A., Fenton, C., and Fa., J. E. 2007. Mountain chickens Leptodactylus fallax and sympatric amphibians appear to be disease free on Montserrat. Oryx 41:398-401.
GarcÌa, G., Lopez, J., Fa, J. E., and Gray, G. A. L. 2009. Chytrid fungus strikes mountain chickens in Montserrat. Oryx 43: 323-328.
Garner, T. W., Perkins, M., Govindarajulu, P., Seglie, D., Walker, S., Cunningham, A. A., and Fisher, M. C. 2006. The emerging amphibian pathogen Batrachochytrium dendrobatidis globally infects introduced populations of the North American bullfrog, Rana catesbeiana. Biology Letters 2: 455ñ459.
Garner, T. W. J., Garcia, G., Carroll, B., and Fisher, M. C. 2009. Using itraconazole to clear Batrachochytrium dendrobatidis infection, and subsequent depigmentation of Alytes muletensis tadpoles. Diseases of Aquatic Organisms 83: 257-260.
Garner, T. W. J., Walker, S., Bosch, J., Hyatt, A. D., Cunningham, A. A., and Fisher, M. C. 2005. Chytrid fungus in Europe. Emerging Infectious Diseases 11: 1639-1641. Available online.
Gleason, F. H., Letcher, P. M., and McGee, P. A. 2004. Some Chytridiomycota in soil recover from drying and high temperatures. Mycological Research 108: 583ñ589.
Goka, K., Yokoyama, J., Une, Y., Kuroki, T., Suzuki, K., Nakahara, M., Kobayashi, A., Inaba, S., Mizutani, T., and Hyatt, A. D. 2009. Amphibian chytridiomycosis in Japan: distribution, haplotypes, and possible route of entry into Japan. Molecular Ecology 18: 4757-4774.
Guayasamin, J. M., Bonaccorso, E. A., Speare, R., and Mendez, D. The roles of climatic variation and pathogenic fungus in declining populations of Atelopus cruciger (Anura: Bufonidae) in Venezuela. Joint Meeting of the American Society of Ichthyologists and Herpetologists, Herpetologists' League, and Society for the Study of Amphibians and Reptiles, 4-8 July 2002, Kansas City, USA.
Gurdon, J. B., and Hopwood, N. 2000. The introduction of Xenopus laevis into developmental biology: of empire, pregnancy testing and ribosomal genes. International Journal of Developmental Biology 44: 43-50.
Hale, S. F., Rosen, P. C., Jarchow, J. L., and Bradley, G. A. 2005. Effects of the chytrid fungus on the Tarahumara Frog (Rana tarahumarae) in Arizona and Sonora, Mexico. in Connecting Mountain Islands and Desert Seas: Biodiversity and Management of the Madrean Archipelago II. Proceedings of the 5th Conference on Research and Resource Management in the Southwestern Deserts, May 11-15, 2004, Tucson, Arizona.
Halliday, T. R. 1998. A declining amphibian conundrum. Nature 394: 418-419.
Hanselmann, R., RodrÌguez, A., Lampo, M., Fajardo-Ramos, L., Aguirre, A., Kilpatrick, A. M., Rodryguez, J. P., and Daszak, P. 2004. Presence of an emerging pathogen of amphibians in introduced bullfrogs Rana catesbeiana in Venezuela. Biological Conservation, 120: 115-119.
Hardman, C. 2001. The potential impact of the chytrid fungus on Tasmania's frog populations. BSc (Honours) thesis, University of Tasmania.
Harris, R. N., Brucker, R. M., Walke, J. B., Becker, M. H., Schwantes, C. R., Flaherty, D. C., Lam, B. A., Woodhams, D. C., Briggs, C. J., Vredenburg, V. T., and Minbiole, K. P. C. 2009a. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. The ISME Journal 3: 818-824.
Harris, R. N., James, T. Y., Lauer, A., Simon, M. A., and Patel, A. 2008. The amphibian pathogen Batrachochytrium dendrobatidis is inhibited by the cutaneous bacteria of amphibian species. EcoHealth 3: 53-56.
Harris, R. N., Lauer, A., Simon, M. A., Banning, J. L., and Alford, R. A. 2009b. Addition of antifungal skin bacteria to salamanders ameliorates the effects of chytridiomycosis. Diseases of Aquatic Organisms 83: 11-16.
Herrera, R. A., Steciow, M. M., and Natale, G. S. 2005. Chytrid fungus parasitizing the wild amphibian Leptodactylus ocellatus (Anura: Leptodactylidae) in Argentina. Diseases of Aquatic Organisms 64: 247-252.
Heyer, W. R., Rand, A. S., GonÁalvez da Cruz, C. A., and Peixoto, O. L. 1988. Decimations, extinctions, and colonizations of frog populations in southeast Brazil and their evolutionary implications. Biotropica 20: 230-235.
Ingram, G. J., and McDonald, K. R. 1993. An update on the decline of Queensland's frogs. Herpetology in Australia: A diverse discipline. D. Lunney and D. Ayers, eds., Transactions of the Royal Zoological Society of New South Wales, 297-303.
James, T. Y., Litvintseva, A. P., Vilgalys, R., Morgan, J. A. T., Taylor, J. W., Fisher, M. C., Berger, L., Weldon, C., du Preez, L., and Longcore, J. E. 2009. Rapid global expansion of the fungal disease chytridiomycosis into declining and healthy amphibian populations. PLoS Pathogens 5(5): e1000458.
James, T. Y., Porter, D., Leander, C. A., Vilgalys, R., and Longcore, J. E. 2000. Molecular phylogenetics of the Chytridiomycota supports the utility of ultrastructural data in chytrid systematics. Canadian Journal of Botany 78: 1-15.
Jancovich, J. K., Mao, J., Chinchar, V. G., Wyatt, C., Case, S. T., Kumar, S., Valente, G., Subramanian, S., Davidson, E. W., Collins, J. P., and Jacobs, B. L. 2003. Genomic sequence of a ranavirus (family Iridoviridae) associated with salamander mortalities in North America. Virology 316: 90-103.
Johnson, M. L., Berger, L., Philips, L., and Speare, R. 2003. Fungicidal effects of chemical disinfectants, UV light, desiccation and heat on the amphibian chytrid Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms 57: 255-260. Johnson, M. L., and Speare, R. 2003. Survival of Batrachochytrium dendrobatidis in water: quarantine and disease control implications. Emerging Infectious Diseases 9: 922-925. Available online. Johnson, M. L., and Speare, R. 2005. Possible modes of dissemination of the amphibian chytrid Batrachochytrium dendrobatidis in the environment. Diseases of Aquatic Organisms 65: 181-186.
Kriger, K. M., and Hero, J. M. 2007. Large-scale seasonal variation in the prevalence and severity of chytridiomycosis. Journal of Zoology 271: 352ñ359.
Kusrini, M. D., Skerratt, L. F., Garland, S., Berger, L., and Endarwin, W. 2008. Chytridiomycosis in frogs of Mount Gede Pangrango, Indonesia. Diseases of Aquatic Organisms 82: 187-194.
La Marca, E., Lips, K. R., Lˆtters, S., Puschendorf, R., IbaÒez, R., Rueda-Almonacid, J. V., Schulte, R., Marty, C., Castro, F., Manzanilla-Puppo, J., GarcÌa-PÈrez, J. E., BolaÒos, F., Chaves, G., Pounds, J. A., Toral, E., and Young, B. E. 2005. Catastrophic population declines and extinctions in neotropical Harlequin Frogs (Bufonidae: Atelopus). Biotropica 37: 190-201.
La Marca, E., and Reinthaler, H. P. (1991). Population changes in Atelopus species of the Cordillera de Merida, Venezuela. Herpetological Review, 22, 125-128.
Lamirande, E. W., and Nichols, D. K. 2002. Effects of host age on susceptibility to cutaneous chytridiomycosis in blue-and-yellow poison dart frogs (Dendrobates tinctorius). Proceeding of the Sixth International Symposium on the Pathology of Reptiles and Amphibians. R. G. McKinnell and D. L. Carlson, eds. Saint Paul, Minnesota.
Laurance, W. F., McDonald, K. R., and Speare, R. 1996. Epidemic disease and the catastrophic decline of Australian rainforest frogs. Conservation Biology 10: 406-413.
Lehtinen, R. M., Kam, Y.-C., and Richards, C. L. 2008. Preliminary surveys for Batrachochytrium dendrobatidis in Taiwan. Herpetological Review 39: 317ñ318.
Lips, K. R. 1998. Decline of a tropical montane amphibian fauna. Conservation Biology 12: 1-13.
Lips, K. R. 1999. Mass mortality and population declines of anurans at an upland Site in Western Panama. Conservation Biology 13: 117-125.
Lips, K. R., Brem, F., Brenes, R., Reeve, J. D., Alford, R. A., Voyles, J., Carey, C., Livo, L., Pessier, A. P., and Collins, J. C. 2006. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proceedings of the National Academy of Sciences of the United States of America 103: 3165-3170.
Lips, K. R., Diffendorfer, J., Mendelson, J. R. III, and Sears, M. W. 2008. Riding the wave: reconciling the roles of disease and climate change in amphibian declines. PLoS Biology 6: 441-454.
Lips, K. R., Green, D. E., and Papendick, R. 2003a. Chytridiomycosis in wild frogs from southern Costa Rica. Journal of Herpetology 37: 215ñ218.
Lips, K. R., Reeve, J. D., and Witters, L. 2003b. Ecological traits predicting amphibian population declines in Central America. Conservation Biology 17: 1078ñ1088.
Longcore, J. E., Pessier, A. P., and Nichols, D. K. 1999. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91: 219-227.
Longo, A. V., and Burrowes, P. A. 2010. Persistence with chytridiomycosis does not assure survival of direct-developing frogs. EcoHealth, published online June 29, 2010, DOI: 10.1007/s10393-010-0327-9.
Lˆtters, S. 2007. The fate of the Harlequin Toads ó help through a synchronous multi-disciplinary approach and the IUCN ëAmphibian Conservation Action Planí. Zoosystematics and Evolution, 83 (Supplement 1): 69-73.
Luger, M., Garner, T. W. J., Ernst, R., Hˆdl, W., and Lˆtters, S. 2008. No evidence for precipitous declines of harlequin frogs (Atelopus) in the Guyanas. Studies on Neotropical Fauna & Environment 43: 177-180.
Mahony, M., Tyler, M.J., and Davies, M. 1984. A new species of the genus Rheobatrachus (Anura: Leptodactylidae) from Queensland. Transactions of the Royal Society of South Australia, 108(3): 155-162.
Marantelli, G., Berger, L., Speare, R., and Keegan, L. 2004. Distribution of the amphibian chytrid Batrachochytrium dendrobatidis and keratin during tadpole development. Pacific Conservation Biology 10: 173-179.
MartÌnez-Solano, I., Bosch, J. and GarcÌa-ParÌs, M. 2003. Demographic trends and community stability in a montane amphibian assemblage. Conservation Biology, 17: 238ñ244.
Mazzoni, R., Cunningham, A. A., Daszak, P., Apolo, A., Perdomo, E., and Speranza, G. 2003. Emerging pathogen of wild amphibians in frogs (Rana catesbeiana farmed for international trade. Emerging Infectious Diseases 9: 995-998. Available online. McCracken, S., Gaertner, J. P., Forstner, M. R. J., and Hahn, D. 2009. Detection of Batrachochytrium dendrobatidis in amphibians from the forest floor to the upper canopy of an Ecuadorian Amazon lowland rainforest. Herpetological Review 40: 190-195.
McCranie, J., and Wilson, L. D. 2002. The Amphibians of Honduras. Contributions to Herpetology, Society for the Study of Amphibians and Reptiles. McDonald, K. R. 1990. Rheobatrachus Liem and Taudactylus Straughan and Lee (Anura: Leptodactylidae) in Eungella National Park, Queensland: distribution and decline. Transactions of the Royal Society of South Australia 114(4): 187-194.
McDonald, K. and Alford, R. 1999. A review of declining frogs in northern Queensland. Declines and Disappearances of Australian Frogs. A. Campbell, ed., Environment Australia, Canberra. Available in .pdf format online. McDonald, K. R., Mendez, D., Muller, R., Freeman, A. B., and Speare, R. 2005. Decline in the prevalence of chytridiomycosis in frog populations in North Queensland, Australia. Pacific Conservation Biology 11: 114ñ120.
McDonald, K. R., and Speare, R. 2000. Banana frogs: what's in the box? Fourth Australian Banana Industry Congress, Cairns, Australia.
McDiarmid, R., and Altig, R. (eds.). 1999. Tadpoles: the biology of anuran larvae. The University of Chicago Press, Chicago.
McIntyre, S. 2003. The current status of the mountain chicken Leptodactylus fallax on Dominica, eastern Caribbean: an amphibian in decline. MSc. Thesis, University of East Anglia, Norwich, UK.
McLeod, D. S., Sheridan, J. A., Jiraungkoorskul, W., and Khonsue, W. 2008. A survey for chytrid fungus in Thai amphibians. Raffles Bulletin of Zoology 56: 199-204.
Miller, C. E., and Dylewski, D. P. 1981. Syngamy and resting body development in Chytriomyces hyalinus (Chytridiales). American Journal of Botany 68: 342ñ349.
Mohneke, M., and Rˆdel, M.-O. 2009. Declining amphibian populations and possible ecological consequences ñ a review. Salamandra 45: 203-210.
Morehouse, E. A., James, T. Y., Ganley, A. R. D., Vilgalys, R., Berger, L., Murphy, P. J., and Longcore, J. E. 2003. Multilocus sequence typing suggests the chytrid pathogen of amphibians is a recently emerged clone. Molecular Ecology 12: 395-403.
Morell, V. 1999. Are pathogens felling frogs? Science 284: 728-731.
Morgan, J. A. T., Vredenburg, V. T., Rachowicz, L. J., Knapp, R. A., Stice, M. J., Tunstall, T., Bingham, R. E., Parker, J. M., Longcore, J. E., Moritz, C., Briggs, C. J., and Taylor, J. W. 2007. Population genetics of the frog killing fungus Batrachochytrium dendrobatidis. Proceedings of the National Academy of Sciences 104(34): 13845-13850. Available online.
Muths, E., Corn, P. S., Pessier, A. P., and Green, D. E. 2003. Evidence for disease-related amphibian decline in Colorado. Biological Conservation 110: 357ñ365.
Mutschmann, F., Berger, L., Zwart, P., and Gaedicke, C. 2000. Chytridiomycosis in amphibians - first report in Europe. Berl Munch Tierarztl 113: 380-383.
Nichols, D. K., Lamirande, E. W., Pessier, A. P., and Longcore, J. E. 2001. Experimental transmission of cutaneous chytridiomycosis in dendrobatid frogs. Journal of Wildlife Diseases 37: 1-11.
Obendorf, D. L. 2005. Developing field & diagnostic methods to survey for chytridiomycosis in Tasmanian frogs. Central North Field Naturalists, Inc. Tasmania, Australia. Report to the Department of Environment and Heritage, Canberra. Available online.
Obendorf, D., and Dalton, A. 2006. A survey for the presence of the amphibian chytrid fungus (Batrachochytrium dendrobatidis) in Tasmania. Papers and Proceedings of the Royal Society of Tasmania 140: 25-29.
O'Dwyer, W. T., Buttemer, W. A., and Priddel, D. M. 2000. Inadvertent translocation of amphibians in the shipment of agricultural produce into New South Wales: its extent and conservation implications. Pacific Conservation Biology 6:40-45. Olsen, V., Hyatt, A. D., Boyle, D. G., and Mendez, D. 2004. Co-localisation of Batrachochytrium dendrobatidis and keratiin for enhanced diagnosis of chytridiomycosis in frogs. Diseases of Aquatic Organisms 61:85-88.
Ouellet, M., Mikaelian, I., Paul, B. D., Rodrigue, J., and Green, D. M. 2005. Historical evidence of widespread chytrid infection in North American amphibian populations. Conservation Biology 19: 1431-1440.
Padgett-Flohr, G. E., and Hopkins, R. L. 2009. Batrachochytrium dendrobatidis, a novel pathogen approaching endemism in central California. Diseases of Aquatic Organisms 83: 1-9.
Parmesan, C. 2006. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology and Systematics 37: 637-669.
Parker, J. M., Mikaelian, I., Hahn, N., and Diggs, H. E. 2002. Clinical diagnosis and treatment of epidermal chytridiomycosis in African clawed frogs (Xenopus tropicalis). Comparative Medicine 52: 265-268.
Parra-Olea, G., Garcia-Paris, M., and Wake, D. B. 1999. Status of some populations of Mexican salamanders (Amphibia: Plethodontidae). Revista de BiologÌa Tropical 47: 217-223.
Parris, M. J. 2004. Hybrid response to pathogen infection in interspecific crosses between two amphibian species (Anura: Ranidae). Evolutionary Ecology Research 6: 457ñ471.
Pauza, M., and Driessen, M. 2008. Distribution and potential spread of amphibian chytrid fungus Batrachochytrium dendrobatidis in the Tasmanian Wilderness World Heritage Area. Report on Amphibian Chytrid Fungus in the TWWHA, Department of Primary Industries, Parks, Water, and Environment. Available online.
Pearl, C. A., Bull, E. L., Green, D. E., Bowerman, J., Adams, M. J., Hyatt, A., and Wente, W. H. 2007. Occurrence of the amphibian pathogen Batrachochytrium dendrobatidis in the Pacific Northwest. Journal of Herpetology 41: 145-149.
Phillott, A. D., Garland, S., and Skerratt, L. F. 2009. Eastern Water Dragons (Physignathus lesueureii) are not important alternate hosts of the frog chytrid fungus Batrachochytrium dendrobatidis. Herpetological Conservation and Biology 4: 379-383.
Picco, A. M., and Collins, J. P. 2008. Amphibian commerce as a likely source of pathogen pollution. Conservation Biology 22: 1582-1589.
Piotrowski, J. S., Annis, S. L., and Longcore, J. E. 2004. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96: 9-15.
Plehn, M. 1916. Pathogene Schimmelpilze in der Fischniere. Zeitschrift f¸r Fischerei 18: 51-54.
Pough, F. H., R. M. Andrews, J. E. Cadle, M. L. Crump, A. H. Savitzky, and K. D. Wells. Herpetology, Third Edition. Pearson Prentice Hall, Upper Saddle River, NJ, USA.
Puschendorf, R., BolaÒos, F., and Chaves, G. 2006. The amphibian chytrid fungus along an altitudinal transect before the first reported declines in Costa Rica. Biological Conservation 132: 136-142.
Puschendorf, R., CastaÒeda, F., and McCranie, J. R. 2006. Chytridiomycosis in wild frogs from Pico Bonito National Park, Honduras. EcoHealth 3: 178-181.
Rachowicz, L. J., Knapp, R. A., Morgan, J. A. T., Stice, M. J., Vredenburg, V. T., Parker, J. M., and Briggs, C. J. 2006. Emerging infectious disease as a proximate cause of amphibian mass mortality. Ecology 87(7): 1671-1683. Available online.
Rachowicz, L. J., and Vredenburg, V. T. 2004. Transmission of Batrachochytrium dendrobatidis within and between amphibian life stages. Diseases of Aquatic Organisms 61: 75-83. Available online.
Raphael, B., and Pramuk, J. 2007. Treatment of chytrid infection in Typhlonectes spp. using elevated water temperatures. Proceedings of the IRCEB meeting, Phoenix, Arizona, November 2007. Unpublished.
Raxworthy, C. J., Pearson, R. G., Rabibisoa, N., Rakotondrazafy, A. M., Ramanamanjato, J.-B., Raselimanana, A. P., Wu, S., Nussbaum, R. A., and Stone, D. A. 2008. Extinction vulnerability of tropical montane endemism from warming and upslope displacement: a preliminary appraisal for the highest massif in Madagascar. Global Change Biology 14: 1703-1720.
Reading, C. J. 2007. Linking global warming to amphibian declines through its effects on female body condition and survivorship. Oecologia 151: 125-131.
Reed, K. D., Ruth, G. R., Meyer, J. A., and Shukla, S. K. 2005. Chlamydia pneumoniae infection in a breeding colony of African Clawed Frogs (Xenopus tropicalis). 2000. Emerging Infectious Diseases 6: 196-199.
Reichle, S. 2006. Distribution, diversity and conservation status of Bolivian amphibians. Ph.D. dissertation, Rheinische Friedrichs-Wilhelm Universit‰t, Bonn.
Retallick, R., McCallum, H., and Speare, R. 2004. Endemic infection of the amphibian chytrid fungus in a frog community post-decline. PLOS Biology 2: 1-7.
Retallick, R. W. R., and Miera, V. 2007. Strain differences in the amphibian chytrid Batrachochytrium dendrobatidis and non-permanent, sub-lethal effects of infection. Diseases of Aquatic Organisms 75: 201-207.
Retallick, R. W. R., Miera, V., Richards, K. L., Field, K. J., and Collins, J. P. 2006. A non-lethal technique for detecting the chytrid fungus Batrachochytrium dendrobatidis on tadpoles. Diseases of Aquatic Organisms 72: 77-85.
Ricardo, H. 2006. Distribution and ecology of chytrid in Tasmania (Honours Thesis). University of Tasmania, Hobart.
Richards, S. J., McDonald, K. R., and Alford, R. A. 1993. Declines in populations of Australia's endemic rainforest frogs. Pacific Conservation Biology 1: 66-77.
Richards-Zawacki, C. L. 2009. Thermoregulatory behaviour affects prevalence of chytrid fungal infection in a wild population of Panamanian golden frogs. Proceedings of the Royal Society B, published online 28 October 2009, doi: 10.1098/rspb.2009.1656.
RodrÌguez, J. P. and Rojas-Su·rez, F. 1995. Libro Rojo De La Fauna Venezolana. PROVITA, Caracas.
RodrÌguez-Contreras, A., Celsa SeÒaris, J., Lampo, M., and Rivero, R. 2008. Rediscovery of Atelopus cruciger (Anura: Bufonidae): current status in the Cordillera de La Costa, Venezuela. Oryx 42: 301-304.
Ron, S. R. 2005. Predicting the distribution of the amphibian pathogen Batrachochytrium dendrobatidis in the New World. Biotropica 37: 209-221.
Ron, S. R., and Merino, A. 2000. Amphibian declines in Ecuador: overview and first report of chytridiomycosis from South America. Froglog 42: 2ñ3.
Rosenblum, E. 2009. Bd genomics updates. Talk presented at the IRCEB Annual Meeting on Emerging Wildlife Diseases: Threats to Amphibian Biodiversity. November 13-14, 2009, Arizona State University.
Rosenblum, E. B., Stajich, J. E., Maddox, N., and Eisen, M. B. 2008. Global gene expression profiles for life stages of the deadly amphibian pathogen Batrachochytrium dendrobatidis. Proceedings of the National Academy of Sciences 105: 17034-17039. Available online.
Rovito, S. M., Parra-Olea, G., V·squez-Almaz·n, C. R., Papenfuss, T. J., and Wake, D. B. 2009. Dramatic declines in neotropical salamander populations are an important part of the global amphibian crisis. Proceedings of the National Academy of Sciences 106: 3231-3236. Available online.
Rowley J. J. L., Chan, S. K. F., Tang, W. S., Speare, R., Skerratt, L. F., Alford, R. A., Cheung, K. S., Ho, C. Y., and Campbell, R. 2007. Survey for the amphibian chytrid Batrachochytrium dendrobatidis in Hong Kong in native amphibians and in the international amphibian trade. Diseases of Aquatic Organisms 78: 87ñ95.
Ruiz, A., and Rueda-Almonacid, J. V. 2008. Batrachochytrium dendrobatidis and chytridiomycosis in anuran amphibians of Colombia. EcoHealth 5: 27-33.
Sch‰perclaus, W., Kulow, H., and Schreckenbach, K. (eds.) 1991. Fish Diseases, Volume 1. (Translation of: Fischkrankheiten.) Akademic-Verlag, Berlin (original version published in German in 1986).
Scherwinski, K., Grosch, R., and Berg, G. 2008. Effect of bacterial antagonists on lettuce: active biocontrol of Rhizoctonia solani and negligible, short-term effects on nontarget microorganisms. FEMS Microbiol Ecol 64: 106ñ116.
Schloegel, L. M., Daszak, P., Cunningham, A. A., Speare, R., and Hill, B. 2010. Two amphibian diseases, chytridiomycosis and ranaviral disease, are now globally notifiable to the Organization for Animal Health (OIE): an assessment. Diseases of Aquatic Organisms, published online January 13, 2010. DOI: 10.3354/dao02140.
Schloegel, L. M., Hero, J. M., Berger, L., Speare, R., McDonald, K., and Daszak, P. 2005. The decline of the sharp-snouted day frog (Taudactylus acutirostris): the first documented case of extinction by infection in a free-ranging wildlife species? EcoHealth 3: 35-40.
Schloegel, L. M., Picco, A. M., Kilpatrick, A. M., Davies, A. J., Hyatt, A. D., and Daszak, P. 2009. Magnitude of the US trade in amphibians and presence of Batrachochytrium dendrobatidis and ranavirus infection in imported North American bullfrogs (Rana catesbeiana). Biological Conservation 142: 1420-1426.
Seimon, T. A., Hoernig, G., Sowell, P., Halloy, S., and Seimon, A. 2005. Identification of chytridiomycosis in Telmatobius marmoratus at 4450 m in the Cordillera Vilcanota of Southern Peru. Monographs in Herpetology 7: 273-281.
Seimon, T. A., Seimon, A., Daszak, P., Halloy, S. R. P., Schloegel, L. M., Aguilar, C. A., Sowell, P., Hyatt, A. D., Konecky, B., and Simmons, J. E. 2006. Upward range extension of Andean anurans and chytridiomycosis to extreme elevations in response to tropical deglaciation. Global Change Biology 12: 1-12.
Skerratt, L. F., Berger, L., Speare, R., Cashins, S., McDonald, K. R., Phillott, A. D., Hines, H. B., and Kenyon, N. 2007. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4: 125-134.
SolÌs, R., Lobos, G., Walker, S. F., Fisher, M., and Bosch, J. 2010. Presence of Batrachochytrium dendrobatidis in feral populations of Xenopus laevis in Chile. Biological Invasions 12: 1641-1646.
Soto-Azat, C., Clarke, B. T., Fisher, M. C., Walker, S. F., and Cunningham, A. A. 2009. Non-invasive sampling methods for the detection of Batrachochytrium dendrobatidis in archived amphibians. Diseases of Aquatic Organisms 84: 163-166.
Soto-Azat, C., Clarke, B. T., Poynton, J. C., and Cunningham, A. A. 2010. Widespread historical presence of Batrachochytrium dendrobatidis in African pipid frogs. Diversity and Distributions 16: 126-131.
Sparrow, F. K. 1960. Aquatic Phycomycetes, 2nd revised edition. University of Michigan Press, Ann Arbor. Speare, R. and Berger, L. 2005. Chytridiomycosis in amphibians in Australia. www.jcu.edu.au/school/phtm/PHTM/frogs/chyspec.htm
Stagni, G., Scoccianti, C., and Fusini, R. 2002. Segnalazione di chytridiomicosi in popolazioni di Bombina pachypus (Anura, Bombinatoridae) dellíAppennino toscoemiliano. Abstracts IV; Congresso della Societas Herpetologica Italica; Napoli: Societas Herpetologica Italica; 2002.
Stockwell, M. P., Clulow, J., and Mahony, M. J. 2010. Efficacy of SYBR 14/propidium iodide viability stain for the amphibian chytrid fungus Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms 88: 177-181.
Toledo, L. F., Britto, F. B., Ara˙jo, O. G. S., Giasson, L. M. O., and Haddad, C. F. B. 2006. The occurrence of Batrachochytrium dendrobatidis in Brazil and the inclusion of 17 new cases of infection. South American Journal of Herpetology 1: 185-191.
Une, Y., Kadekaru, S., Tamukai, K., Goka, K., and Kuroki, T. 2008. First report of spontaneous chytridiomycosis in frogs in Asia. Diseases of Aquatic Organisms 82: 157-160.
Van Ells, T., Stanton, J., Strieby, A., Daszak, P., Hyatt, A. D., and Brown, C. 2003. Use of immunohistochemistry to diagnose chytridiomycosis in dyeing poison dart frogs (Dendrobates tinctorius). Journal of Wildlife Diseases 39: 742-745.
Van Sluys, M., and Hero, J.-M. 2010. How does chytrid infection vary among habitats? The case of Litoria wilcoxii (Anura, Hylidae) in SE Queensland, Australia. EcoHealth, DOI: 10.1007/s10393-010-0278-1.
Vazquez, V. M., Rothermel, B. B., and Pessier, A. P. 2009. Experimental infection of North American plethodontid salamanders with the fungus Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms 84: 1-7.
Vredenburg, V. T., Knapp, R. A., Tunstall, T. S., and Briggs, C. J. 2010. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proceedings of the National Academy of Sciences 107: 9689-9694. Available online.
Voyles, J., Berger, L., Young, S., Speare, R., Webb, R., and Warner, J. 2007. Electrolyte depletion and osmotic imbalance in amphibians with chytridiomycosis. Diseases of Aquatic Organisms 77: 113-118.
Voyles, J., Young, S., Berger, L., Campbell, C., Voyles, W. F., Dinudom, A., Cook, D., Webb, R., Alford, R. A., Skerratt, L. F., and Speare, R. 2009. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 326: 582-585.
Waldman, B., van de Wolfshaar, K. E., Klena, J. D., Andjic, V., Bishop, P. J. and Norman, R. J. de B., 2001. Chytridiomycosis in New Zealand frogs. Surveillance 28: 9ñ11.
Walker, S., Bosch, J., James, T. Y., Litvintseva, A., Valls, J. A. O., PiÒa, S., Garcia, G., Abadie Rosa, G., Cunningham, A. A., Hole, S., Griffiths, R., and Fisher, M. C. 2008. Invasive pathogens threaten species recovery programs. Current Biology 18: R853-R854.
Wei, Y., Xu, K., Zhu, D.-Z., Chen, X.-F., and Wang, X.-L. 2010. Early-spring survey for Batrachochytrium dendrobatidis in wild Rana dybowskii in Heilongjiang Province, China. Diseases of Aquatic Organisms, published online February 17, 2010, DOI 10.3354/dao02172.
Weinstein, S. 2009. An aquatic disease on a terrestrial salamander: individual and population level effects of the amphibian chytrid fungus, Batrachochytrium dendrobatidis, on Batrachoseps attenuatus (Plethodontidae). Copeia 2009 (4): 653-660.
Weldon, C. 2005. Chytridiomycosis, an emerging infectious disease of amphibians in South Africa. Ph.D. dissertation, North-West University, Potschefstroom.
Weldon, C. and du Preez, L.H. 2004. Decline of the Kihansi Spray Toad, from the Udzungwa Mountains, Tanzania. Froglog 62: 2-3.
Weldon, C., du Preez, L. H., Hyatt, A. D., Muller, R., and Speare, R. 2004. Origin of the amphibian chytrid fungus. Emerging Infectious Diseases 10: 2100-2105. Available online.
Weldon, C., du Preez, L., and Vences, M. 2008. Lack of detection of the amphibian chytrid fungus (Batrachochytrium dendrobatidis) in Madagascar. In: A Conservation Strategy for the Amphibians of Madagascar. Monografia XLV, Museo Regionale di Scienze Naturali, Torino.
Weygoldt, P. 1989. Changes in the composition of mountain stream frog communities in the Atlantic mountains of Brazil: frogs as indicators of environmental deteriorations? Studies of Neotropical Fauna and Environment 243: 249-255.
Wilson, L. D., and McCranie, J. R. 2004. The herpetofauna of Parque Nacional Cusuco, Honduras (Reptilia, Amphibia). Herpetological Bulletin 87: 13-24.
Winter, J., and McDonald, K. 1986. Eungella, the land of cloud. Australian Natural History 22: 39-43.
Wood, L. R., Griffiths, R. A., and Shley, L. 2009. Amphibian chytridiomycosis in Luxembourg. Bulletin de la SociÈtÈ des Naturalistes Luxembourgeois 110: 109-114.
Woodhams, D. C., and Alford, R. A. 2005 Ecology of chytridiomycosis in rainforest stream frog assemblages of tropical Queensland. Conservation Biology 19: 1449ñ1459.
Woodhams, D. C., Bosch, J., Briggs, C. J., Cashins, S., Davis, L. R., Lauer, A., Muths, E., Puschendorf, R., Schmidt, B. R., Sheafor, B., and Voyles, J. 2011. Mitigating amphibian disease: strategies to maintain wild populations and control chytridiomycosis.
Woodhams, D. C., Vredenburg, V. T., Simon, M. A., Billheimer, D., Shakhtour, B., Shyr, Y., Briggs, C. J., Rollins-Smith, L. A., and Harris, R. N. (2007). Symbiotic bacteria contribute to innate immune defenses of the threatened mountain yellow-legged frog, Rana muscosa. Biological Conservation 138: 390ñ398. Available online.
Yang, H., Baek, H., Speare, R., Webb, R., Park, S., Kim, T., Lasater, K. C., Shin, S, Son, S., Park, J., Min, M., Kim, Y., Na, K., Lee, H., and Park, S. 2009. First detection of the amphibian chytrid fungus Batrachochytrium dendrobatidis in free-ranging populations of amphibians on mainland Asia: survey in South Korea. Diseases of Aquatic Organisms 86: 9ñ13.
Young, S., Berger, L., and Speare, R. 2007. Amphibian chytridiomycosis: strategies for captive management and conservation. International Zoo Yearbook 41: 1-11.
Written by Kellie Whittaker (1) and Vance Vredenburg (2). (1) Museum of Vertebrate Zoology, University of California, Berkeley
(2) Department of Biology, San Francisco State University Last updated: May 17, 2011.
No comments:
Post a Comment