Monday, 31 August 2015
I hope this all makes sense, So far so good
As I realign it becomes very apparent that the use of gravity affects our every move from pulling our socks up to taking a lid off of a yoghurt pot. Our hand constantly adapts to our every move, the complexities of it are amazing, when you lean forward the hand opens slightly, it's so subtle. Now my neck is much stronger and can hold the weight of my head I can lean from side to side forward or back and I go naturally back to centre with no effort at all. Our hands are constantly used to keep us balanced by pushing away or pulling us forward. I can sit in my chair and push up and then sit back down. My feet now rest on the floor, I am no longer constantly aware of them. I cannot independently move my legs, but if I pull up on the front of the knee I can move them. For every move we make another part of the body compensates for that.
Tom Volk's fungus of the month in 2002 incl. Valley fever
Tom Volk's Fungus of the Month for January 2002
This month's fungus is Coccidioides immitis, cause of the fungal disease coccidioidomycosis, aka Valley Fever, San Joaquin Valley Fever, desert bumps, desert rheumatism or Posadas' disease
 Coccidioides immitis (kok-sid-ee-OID-eez IMM-ih-tiss) is the cause of a nasty fungal disease called coccidioidomycosis (kok-sid-ee-oid-oh-my-KOH-sis). Like the other true-pathogenic, systemic human fungal diseases histoplasmosis, blastomycosis, and paracoccidioidomycosis, Coccidioidomycosis starts out as a lung disease caused by inhalation of the conidia, shown to the left. Most often the disease causes mild flu-like symptoms, but usually is resolved in the lungs.
Coccidioides immitis (kok-sid-ee-OID-eez IMM-ih-tiss) is the cause of a nasty fungal disease called coccidioidomycosis (kok-sid-ee-oid-oh-my-KOH-sis). Like the other true-pathogenic, systemic human fungal diseases histoplasmosis, blastomycosis, and paracoccidioidomycosis, Coccidioidomycosis starts out as a lung disease caused by inhalation of the conidia, shown to the left. Most often the disease causes mild flu-like symptoms, but usually is resolved in the lungs.  This fungus is a dimorphic pathogen, which means it can change from the room-temperature hyphal form at to the body-temperature spherule form (shown to the right) containing endospores. These endospores can be transported by the bloodstream to other parts of the body, particularly to the brain and central nervous system, where they can germinate and grow to cause even more severe disease. The dimorphism helps the fungus to evade the immune system by the changing of the surface antigens of the fungus.
This fungus is a dimorphic pathogen, which means it can change from the room-temperature hyphal form at to the body-temperature spherule form (shown to the right) containing endospores. These endospores can be transported by the bloodstream to other parts of the body, particularly to the brain and central nervous system, where they can germinate and grow to cause even more severe disease. The dimorphism helps the fungus to evade the immune system by the changing of the surface antigens of the fungus. 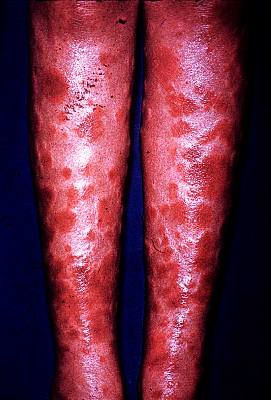 The disease often begins as a benign, inapparent or mildly severe upper respiratory infection that usually resolves rapidly. Recovery from mild forms of the disease usually results in lifelong immunity to reinfection. However, if there are enough spores inhaled, or if the person's immune system is compromised in some way, the disease can spread to other parts of the body, Rarely the disease is an acute or chronic severe disseminating fatal mycosis. If infection is established, the disease may progress as a chronic pulmonary condition or as a systemic disease involving the meninges (lining of the brain), bones, joints, and subcutaneous and cutaneous tissues. Such involvement is characterized by the formation of burrowing abscesses. Although the symptoms of the disease are quite variable, but often the patient has an allergic reaction to the circulating fungus, producing reddening of the skin known as "desert bumps," shown to the left.
The disease often begins as a benign, inapparent or mildly severe upper respiratory infection that usually resolves rapidly. Recovery from mild forms of the disease usually results in lifelong immunity to reinfection. However, if there are enough spores inhaled, or if the person's immune system is compromised in some way, the disease can spread to other parts of the body, Rarely the disease is an acute or chronic severe disseminating fatal mycosis. If infection is established, the disease may progress as a chronic pulmonary condition or as a systemic disease involving the meninges (lining of the brain), bones, joints, and subcutaneous and cutaneous tissues. Such involvement is characterized by the formation of burrowing abscesses. Although the symptoms of the disease are quite variable, but often the patient has an allergic reaction to the circulating fungus, producing reddening of the skin known as "desert bumps," shown to the left. In culture the extremely small arthroconidia are produced on medium in 5-10 days. The mechanism of spore formation is quite different than what you might be used to. Thallic conidiation (formation of spores from pre-formed hyphae) takes place here. Alternate cells increase in turgidity and in wall thickness to become arthroconidia, while the intervening cells gradually lose cytoplasm. Nuclei remain in the degenerating cells until the adjacent spores completely mature. Arthroconidia are barrel or cask shaped, 2-4 mm X 6 mm, although there may be atypical isolates with different shapes. The spherules cannot normally be induced in culture, but require inhalation into an animal. These spherules bear a superficial resemblance to a chicken protozoan parasite Coccidia; Coccidioides literally means "like Coccidia" and immitis means "not mild." Incidentally, the other type of conidiation, far more common, is blastic conidiation. In this type, the spores are produced de novo from the budding out of hyphal tips. See this page on Aspergillus for an example of blastic conidiation. The teleomorph (sexual state, meiotically-reproducing state) of Coccidioides is unknown. The anamorph (asexual state, mitotically-reproducing state) has some characteristics of Zygomycota (spherule development is like sporangium development), except that it is regularly septate, has Woronin bodies near the septa, characteristic of Ascomycota and deuteromycetes. Analysis of 18S rDNA sequence indicates a close phylogenetic relationship of C. immitis and the Ascomycota, especially with the Arthrodermataceae (Onygenales), such as Ajellomyces dermatitidis and A. capsulatus. If this is true, then essentially all the true systemic pathogenic fungi, as well as the dermatophytes, would be classified in that small group of Ascomycota.
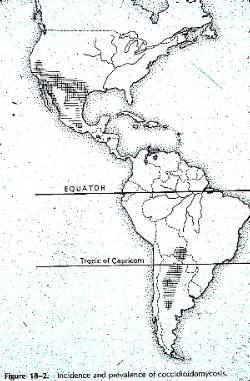 Coccidioides is probably the most virulent of the fungal pathogens. Highly endemic disease areas are semi-arid regions, rather than completely dry. Its occurrence correlates well with the Lower Sonoran Life Zone, which occurs in desert-like areas throughout the southwestern USA and California, as well as into Mexico and Central and South America. It is prevalent in areas that receive rainfall of about 10 inches (25cm) /year occurring all in one season. The west side of the San Joaquin Valley in California is the most endemic area, but even within this region the fungus is highly restricted to a few small areas. However, spores may blow around-- for example a windstorm in 1977 in the San Joaquin Valley blew spores outside endemic area, and 379 new cases of coccidioidomycosis were attributed to the dust storm. Travel can also spread disease. The main problem is with population growth in endemic areas. People not previously exposed move to the area and develop the disease. Some have mild form and acquire immunity; others develop severe disease.
Coccidioides is probably the most virulent of the fungal pathogens. Highly endemic disease areas are semi-arid regions, rather than completely dry. Its occurrence correlates well with the Lower Sonoran Life Zone, which occurs in desert-like areas throughout the southwestern USA and California, as well as into Mexico and Central and South America. It is prevalent in areas that receive rainfall of about 10 inches (25cm) /year occurring all in one season. The west side of the San Joaquin Valley in California is the most endemic area, but even within this region the fungus is highly restricted to a few small areas. However, spores may blow around-- for example a windstorm in 1977 in the San Joaquin Valley blew spores outside endemic area, and 379 new cases of coccidioidomycosis were attributed to the dust storm. Travel can also spread disease. The main problem is with population growth in endemic areas. People not previously exposed move to the area and develop the disease. Some have mild form and acquire immunity; others develop severe disease. The fungus can survive at 20 cm under the soil, but is absent on the surface during hot dry weather. It is also apparently absent from the soil or aerosol during wet winter and spring, but grows again in the summer and becomes aerosolized in late summer and fall. Sept-Nov. is peak endemic period. The favored soils are alkaline, have a high content of carbonized organic materials and a high salt concentration, particularly calcium sulfate and borates. The sterile surface is reinvaded after the rainy season, possibly when capillary action of evaporation reestablishes a favorable salt concentration. Survival of the organism also occurs in rodent burrows. Rodents may become infected also and may be responsible for spreading the organism.
 Occupational hazards are in those occupations in the exposure to soil dust, including agricultural workers, construction workers, telephone pole diggers, and archaeological students. Dark-skinned individuals seem to be more prone to severe infection than Caucasians. AIDS cases limited by geography. Person to person transmission documented in only 5 cases-- 3 were premature infants, one was embalmer who accidentally pricked self while working on a person who had died of the disease. The other case was 6 medical staff members who inhaled arthrospores of Coccidioides growing on a plaster cast of a patient with coccidioidal ostemyelitis. There is a Coccidioides skin test, very similar to the tuberculosis skin test. The test shows that most people in endemic areas have come into contact with the organism. A small fever is usually the only symptom. Most people don't know they have had it. Thus the skin test is of limited diagnostic value since most people in endemic areas will test positive. The disease must be diagnosed from tissue material-- spherules with endospores must be found for an accurate diagnosis. The disease is notoriously difficult to diagnose-- the expert on this fungus Charles E. Smith wrote in "Reminiscences of the Flying Chlamydospore" that he had misdiagnosed his wife's, his son's and *his own* case of coccidioidomycosis. The limerick at the beginning of this page has been attributed to him. Since this is the most virulent of the fungal pathogens, it should never be grown out in culture except under very controlled conditions, such as using gloved transfer hood and in screw cap vials. The fungus produces its small arthrospores in abundance in culture. If these escape, they can cause lab infections. These arthrospores can pass through a 2 mm filter found in normal biological safety cabinets/ hoods! It is a very dangerous organism. There have been persistent rumors that it is being developed for use in biological warfare, but it could probably not be grown in a large enough quantity to be used for use in bioterrorism because of the danger it would pose for the people growing it. Some treatments for systemic coccidioidomycosis include Amphoteracin B or the azoles ketoconazole or itraconazole. Fungal diseases are notoriously difficult to treat because it is difficult to find drugs that kill the fungus without killing the human or animal host. Remember than animals and fungi are rather closely related and have much physiology in common since they are both eukaryotic. It's relatively easy to find ways to kill bacteria causing infections because bacteria are prokaryotic and there are many physiological differences that can be exploited.
Occupational hazards are in those occupations in the exposure to soil dust, including agricultural workers, construction workers, telephone pole diggers, and archaeological students. Dark-skinned individuals seem to be more prone to severe infection than Caucasians. AIDS cases limited by geography. Person to person transmission documented in only 5 cases-- 3 were premature infants, one was embalmer who accidentally pricked self while working on a person who had died of the disease. The other case was 6 medical staff members who inhaled arthrospores of Coccidioides growing on a plaster cast of a patient with coccidioidal ostemyelitis. There is a Coccidioides skin test, very similar to the tuberculosis skin test. The test shows that most people in endemic areas have come into contact with the organism. A small fever is usually the only symptom. Most people don't know they have had it. Thus the skin test is of limited diagnostic value since most people in endemic areas will test positive. The disease must be diagnosed from tissue material-- spherules with endospores must be found for an accurate diagnosis. The disease is notoriously difficult to diagnose-- the expert on this fungus Charles E. Smith wrote in "Reminiscences of the Flying Chlamydospore" that he had misdiagnosed his wife's, his son's and *his own* case of coccidioidomycosis. The limerick at the beginning of this page has been attributed to him. Since this is the most virulent of the fungal pathogens, it should never be grown out in culture except under very controlled conditions, such as using gloved transfer hood and in screw cap vials. The fungus produces its small arthrospores in abundance in culture. If these escape, they can cause lab infections. These arthrospores can pass through a 2 mm filter found in normal biological safety cabinets/ hoods! It is a very dangerous organism. There have been persistent rumors that it is being developed for use in biological warfare, but it could probably not be grown in a large enough quantity to be used for use in bioterrorism because of the danger it would pose for the people growing it. Some treatments for systemic coccidioidomycosis include Amphoteracin B or the azoles ketoconazole or itraconazole. Fungal diseases are notoriously difficult to treat because it is difficult to find drugs that kill the fungus without killing the human or animal host. Remember than animals and fungi are rather closely related and have much physiology in common since they are both eukaryotic. It's relatively easy to find ways to kill bacteria causing infections because bacteria are prokaryotic and there are many physiological differences that can be exploited.  Coccidioidomycosis was discovered by Alejandro Posadas in 1891 in a hospital in Buenos Aries, Argentina. He had a patient named Domingo Ezcurra who had the disease, and Posadas was able to study the progression of the disease over the course of seven years. Although the disease was discovered in Argentina, the second case was not found until 35 years later, and fewer than 30 cases were reported there before 1967. Interestingly, the preserved mortal remains of Domingo Ezcurra were found in 1948 in the anatomy museum of the medical school. His head is now the showpiece of the medical school museum and has been exhibited at medical mycology meetings in South America. In fact this picture was taken at such a meeting by Pat Kammeyer of the Loyola University of Chicago.
Coccidioidomycosis was discovered by Alejandro Posadas in 1891 in a hospital in Buenos Aries, Argentina. He had a patient named Domingo Ezcurra who had the disease, and Posadas was able to study the progression of the disease over the course of seven years. Although the disease was discovered in Argentina, the second case was not found until 35 years later, and fewer than 30 cases were reported there before 1967. Interestingly, the preserved mortal remains of Domingo Ezcurra were found in 1948 in the anatomy museum of the medical school. His head is now the showpiece of the medical school museum and has been exhibited at medical mycology meetings in South America. In fact this picture was taken at such a meeting by Pat Kammeyer of the Loyola University of Chicago. There is some recent work on Coccidioides by John Taylor and his associates at the University of California- Berkeley. They have DNA evidence that there are in fact two species of Coccidioides. One, still to be called Coccidioides immitis, appears to be restricted to California. Specimens found outside of California appear to be a distinct species and have been renamed named Coccidioides posadasii, after Alejandro Posadas. I don't know what, if any, morphological features distinguish the species. You can read the paper here or find it on your own as: Matthew C. Fisher, Gina L. Koenig, Thomas J. White and John W. Taylor. 2002. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia 94(1): 73-84.
By the way, does anyone remember a drum and bugle corps in the 1980's from Modesto, California called Valley Fever? I used to like to watch them. According to an email I got from one of the former members, Anna-Marie Bratton, the corps is kind of starting up again as Fever Drum and Bugle Corps. I wonder how many people who saw the corps knew the origin of their name....?
Thanks to John Rippon, Al Rogers and Tex Beneke (all famous retired medical mycologists!) for their pictures for this month's Fungus of the Month. I hope you enjoyed learning something about Coccidioides immitis today. I hope you manage to avoid personal contact with this particular fungus. It's a nasty one!
If you have anything to add, or if you have corrections or comments, please write to me at volk.thom@uwlax.edu
This page and other pages are © Copyright 2002 by Thomas J. Volk, University of Wisconsin-La Crosse.Return to Tom Volk's Fungi Home Page -- http://TomVolkFungi.net Return to Tom Volk's Fungus of the month pages listing
Postmortem bacteriology - a re-evaluation - 2006
J Clin Pathol. 2006 Jan; 59(1): 1–9.
PMCID: PMC1860254
Postmortem bacteriology: a re‐evaluation
J A Morris, L M Harrison, Department of Pathology, Royal Lancaster Infirmary, Lancaster, LA1 4RP, UK
S M Partridge, Department of Pathology, Furness General Hospital, Barrow in Furness, LA14 4LF, UK
Correspondence to: Professor J A Morris
Department of Pathology, Royal Lancaster Infirmary, Lancaster, LA1 4RP, UK; Jim.A.Morris@rli.mbht.nhs.uk
Department of Pathology, Royal Lancaster Infirmary, Lancaster, LA1 4RP, UK; Jim.A.Morris@rli.mbht.nhs.uk
Accepted 2005 May 19.
Copyright © 2006 The BMJ Publishing Group and the Association of Clinical Pathologists
This article has been cited by other articles in PMC.
Abstract
Aim
To assess the value of postmortem bacteriology in necropsy practice, with specific emphasis on bacterial invasion of blood and cerebrospinal fluid (CSF).
Methods
A review of published articles on postmortem bacteriology. Studies were selected to cover the full range of necropsy practice including adults, the perinatal period, and infancy. The review covers over 5000 necropsies, mainly in adults, but including 1108 perinatal cases and 468 cases of sudden unexpected death in infancy. Data are available on 4992 blood cultures, 1168 specimens of CSF, and 743 cultures of spleen.
Results
Studies in which careful precautions have been taken to reduce contamination show that approximately two thirds of blood cultures are negative, two in nine yield a single isolate, and one in nine have a mixed growth. The postmortem interval has only a small effect on the isolation rate. A pure growth of a known pathogen has a more than 50% likelihood of being found in association with genuine infection in adults and in the perinatal period.
Conclusions
The main postmortem artefact is contamination, but this can be considerably reduced by careful technique. Agonal spread is less common than is often assumed. Postmortem translocation is not a problem if the body is appropriately stored. A pure growth of a pathogen in blood or CSF should be regarded as a possible contributing factor to death at all ages.
Keywords: postmortem bacteriology, agonal spread, postmortem translocation, contamination, sudden unexpected death in infancy
It is difficult to determine the importance of bacteria isolated from a blood or cerebrospinal fluid (CSF) culture obtained at necropsy. In theory, there are several ways in which a positive result can arise.
- Genuine positive: bacteria invade in life and reach the target organ or fluid before death. The mere presence of an organism does not necessarily imply infection and disease because episodes of bacteraemia can occur in life without significant symptoms and without evidence of damage or inflammation. But if bacteraemia occurred before death and was present at death it would be considered to be at least a possible contributing factor. If bacteraemia were to occur in association with symptoms and signs of disease and inflammation then the presence of the organism would be accorded greater importance. A genuinely positive bacterial isolate will usually be a pure growth of a recognised pathogen.
- Agonal spread: bacteria invade during the process of dying or during the time when the circulation is artificially maintained by attempts at resuscitation. The concept is that the integrity of the mucosal surfaces is compromised by ischaemia and/or hypoxia, leading to invasion by several different bacterial species. In this case, bacterial invasion is not a cause of death but a consequence of death. The growth will probably be mixed and include both pathogens (potential) and commensals.
- Postmortem translocation: bacteria migrate from the mucosal surface into the blood and body tissues after death and after the circulation has ceased, but before the necropsy.
- Contamination: the bacteria are introduced into the blood or CSF or organ sample when it is obtained.
Processes 1, 3, and 4 can undoubtedly occur. Bacteria can invade and cause disease; they can also spread in the blood without causing disease; a dead body will putrefy at room temperature as a result of postmortem bacterial growth and tissue invasion; and contamination is a problem when cultures are obtained in life as in death.1,2 However, the process of agonal spread is a theoretical concept, and it might or it might not occur. In the process of dying, the mucosal surfaces are rendered ischaemic and the performance of the immune defences is compromised; this could lead to increased bacterial invasion, but there is no direct evidence that this does in practice occur.
“The process of agonal spread is a theoretical concept, and it might or it might not occur”
Weinstein used a predictive model with multiple variables to assess the results of blood cultures obtained in life.2 It was found that microorganism identity was an independent predictor. Microorganisms that nearly always (> 90%) represent true bacteraemia or fungaemia include Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, other members of the Enterobacteriaceae, Pseudomonas aeruginosa, and Candida albicans. In addition, Streptococcus pyogenes, Streptococcus agalactiae, Listeria monocytogenes, Neisseria meningitidis, Neisseria gonorrhoea, Haemophilus influenzae, members of the Bacteroides fragilis group, candida species other than C albicans, and Cryptococcus neoformans nearly always represent true infection. In contrast, corynebacterium species, bacillus species other than Bacillus anthracis, and Propionibacterium acnes rarely represent true bacteraemia. Coagulase negative staphylococci are also likely to be contaminants unless associated with catheters or vascular prostheses. Enterococci and the viridans group of streptococci can be found as contaminants and as part of a genuine bacteraemia.
Standards issued by the American Society of Microbiology indicate that the rate of blood culture contamination should not exceed 3%, but in practice most hospital contamination rates are in the order of 4–6%.1 It is unlikely that contamination rates as low as this can be achieved in necropsy practice.
In this article, we review several postmortem microbiological studies carried out over the past century. The aim is to try to assess the value of the results in diagnostic practice and to estimate the relative contribution of the above four different mechanisms of entry.
Historical review
In 1916, Fredette reported a series of 119 cases in which blood cultures were obtained within 30 minutes of death.3 Blood was obtained from the median basilic vein using a capillary tube and cultured in plain serum broth. A careful aseptic technique combined with iodine to sterilise the skin minimised contamination. Postmortem dissection of the body was undertaken in all cases and Fredette's paper lists the cause of death and the results of blood culture for each case. Of the 119 cases, 77 had no growth on blood culture (65%), 31 cultures grew a single organism (26%), and 11 cultures produced two organisms (9%). In 56 cases the postmortem anatomical diagnosis indicated an infective cause of death; 31 of these cases had positive blood cultures and 25 had negative cultures. In the 63 cases in which the anatomical diagnosis indicated a non‐infective cause, 11 cultures were positive and 52 were negative. From these figures, it can be calculated that a positive blood culture as a marker for an infective cause of death in this series had a sensitivity of 55%, a specificity of 83%, a positive predictive value (PPV) of 78%, and a negative predictive value (NPV) of 68%.
Fifteen patients died of lobar pneumonia, a diagnosis that can be made with confidence at necropsy. In seven of these patients, S pneumoniae was grown from the blood culture. There were 92 patients in whom the anatomical cause of death was not pneumonia and not meningitis; in two of these, S pneumoniae was isolated from the blood and in 90 it was not. Growth of S pneumoniae as a marker of lobar pneumonia in this series has a sensitivity of 47%, a specificity of 98%, a PPV of 78%, and an NPV of 92%. Furthermore, of the 15 patients with lobar pneumonia, four had organisms in the blood that were not expected and were apparently unrelated to the cause of death (Streptococcus salivarius in three and Staphylococcus epidermidis in one). Thus, in 11 of 15 there was no agonal invasion but in four of 15 some form of contamination had occurred.
Streptococcus pneumoniae was isolated from 14 patients; seven with lobar pneumonia, four with pneumonia, one with meningitis, and two others in which its presence did not correlate with the cause of death. The PPV is 86% (12 of 14 true positives).
Streptococcus pyogenes was isolated in nine cases, eight of which were judged to be true positives on the basis of the anatomical findings (PPV of 89%). Staphylococcus aureus was isolated in six cases, four of which appeared to be true positives (PPV of 67%). Other streptococci were isolated in 11 cases and four of these were possible true positives. Miscellaneous isolates included lactobacilli (two cases), S epidermidis (one case), Corynebacterium pseudodiphtheriticum (one case), Salmonella typhi (one case), and Gram negative bacillus (one case). With the exception of the S typhi, found in a case of typhoid fever, these organisms were probably false positives.
Giordano and Barnes undertook careful bacteriological studies before necropsy in 213 cases.4 They paid scrupulous attention to technique to reduce the possibility of contamination when cultures were obtained. The surface of the appropriate tissue or organ was seared with a red hot spatula and then fluid or tissue was obtained using a pipette plunged through the sterilised area. Material was inoculated into a tube of glucose broth for culture. Time from death to necropsy was one to 23 hours but most of the examinations were conducted in under 12 hours. Blood culture was positive in 80 of 206 cases (38%) and spleen culture was positive in 75 of 190 cases (39%).
Hunt and colleagues obtained blood for culture from the pulmonary artery in 567 postmortem cases.5 The surface of the artery was sterilised by a hot spatula or a flame before obtaining the specimen using a sterile syringe and needle. The postmortem examinations were performed within one to 12 hours of death. One hundred and seventy three cultures were positive (31%).
Burn reported the results of studies in which samples of heart blood, lung, and spleen were obtained from over 136 postmortem cases.6 He also emphasised the importance of taking stringent precautions to avoid contamination. The surface of the relevant organs was sterilised using a heated broad bladed spatula and then sterile scissors were used to obtain samples for culture. The age range was 0 to 90 years, and the interval between death and necropsy was one to 48 hours (recorded for 122 cases); 63 within one to four hours, 24 within five to eight hours, 15 within nine to 12 hours, and 20 within 13 to 48 hours. Heart blood cultures were positive in 49 of 134 cases (37%) yielding 68 isolates. These comprised staphylococci (13 isolates), S pyogenes (10 isolates), S pneumoniae (nine isolates), E coli (eight isolates), proteus group (eight isolates), α haemolytic streptococci (six isolates), non‐haemolytic streptococci (four isolates), Clostridium perfringens (three isolates), S typhi (two isolates), P aeruginosa (two isolates), H influenzae (one isolate), and diphtheroids (one isolate). Spleen cultures were positive in 61 of 136 cases (45%) with a similar range of organisms. Lung cultures were positive in 107 of 121 cases (88%). The percentage of positive cultures did not increase with increasing time from death to necropsy.
“The proportion of positive blood samples increased with postmortem interval from 20% to 40% between 0 and 18 hours”
Burn also conducted experimental studies on postmortem bacterial invasion in guinea pigs and rabbits.7 Bacteria were introduced into the pleural cavity; the animals were then maintained at 10°C, 25°C, or 37°C for two to 96 hours before necropsy. Samples of heart blood, liver, spleen, and kidney were obtained using the same technique as in the human study. Clostridium perfringens, E coli, and staphylococci were capable of invading tissues from five to 48 hours after death if the animal was maintained at 25°C. A wide range of other pathogenic and non‐pathogenic bacteria failed to translocate from the pleura to abdominal organs in these experiments.
Adelson and Kinney studied 126 cases of sudden unexpected death in infancy (SUDI; age range 10 days to 2 years) in which detailed postmortem examinations were conducted and specimens obtained for bacteriology.8 Cultures of heart blood, spinal fluid, pharynx, ileum, and lungs were made in each case. Of 120 heart blood cultures, 95 (79%) were sterile, and the other 25 yielded a total of 32 organisms: E coli (15 isolates), Enterobacter aerogenes (six isolates), S epidermidis (five isolates), S aureus (four isolates), and α haemolytic streptococci (two isolates). The first 38 CSF specimens were sterile and the rest were not examined. They found no relation between the blood culture results and histological findings in the lungs or elsewhere.
Kurtin reported the results of a necropsy study of 50 patients aged 31 to 85 years.9 He used a hot spatula to sterilise the surface of the heart, spleen, and lung before obtaining uncontaminated specimens of heart blood, lung, and splenic tissue. The time from death to necropsy was: less than six hours in nine cases, six to 12 hours in five cases, 12 to 24 hours in 23 cases, and 24 to 48 hours in 13 cases. The heart blood was sterile in 80% and the spleen was sterile in 76%. There was no relation between time to necropsy and sterility. Fourteen of 17 patients with histological evidence of respiratory infection had negative blood and splenic cultures.
Carpenter and Wilkins undertook a retrospective review of 2033 necropsies carried out between 1955 and 1960 in which heart blood cultures and lung cultures had been obtained.10 The technique involved searing the surface of the left ventricle with a soldering iron and then aspirating heart blood for culture. The surface of the lung was also seared in the same way and then a swab was inserted to obtain tissue. Sixty eight percent of the blood cultures were sterile, 25% yielded a single organism, and 7% yielded two or more organisms. The lung cultures were positive in 68%. Heart blood cultures were positive in 46% of paediatric cases, 37% of cases from surgery, 27% of medical cases, and 15% of patients found to be dead on arrival at hospital. The proportion of positive blood cultures increased with length of hospital stay; from 25% to 40% over a 25 day period (correlation coefficient, 0.77; p < 0.01). The proportion positive also increased with postmortem interval from 20% to 40% between 0 and 18 hours (correlation coefficient, 0.74; p < 0.001). Positive lung cultures also increased with length of hospital stay and postmortem interval.
“The introduction of tissue procurement centres with sterile morgue facilities allowed a new method of evaluating human postmortem microbiology”
Wood and colleagues reported 62 necropsy cases in which blood cultures were obtained.11 The age range was 2 months to 90 years, with more than one third over 60 years. At necropsy, the pericardial sac was opened and the surface of the right atrium was seared with a red hot spatula; blood was then aspirated for culture. In this series, blood culture was positive in 44 of 62 cases; 31 cases yielded a single organism on culture, 11 yielded two or more organisms. There were 35 cases in which there was anatomical evidence of infection: 32 of these cases had positive cultures (true positives) and three had negative cultures (false negatives). In 27 cases there was no anatomical evidence of infection; 12 of these cases had positive cultures (false positives) and 15 had negative cultures (true negatives). From these figures one can calculate a sensitivity of 92%, a specificity of 56%, a PPV of 73%, and an NPV of 84%. The authors noted an effect of postmortem interval; the figure for true positives plus true negatives expressed as a percentage was over 83% in cases examined within 15 hours of death but 63% in those examined after 15 hours. The isolates were: S aureus (17 isolates; 13/four (13 judged to be true positives and four false positives)), E coli (11 isolates; 11/0), enterobacter (eight isolates; four/four), proteus (seven isolates; six/one), pseudomonas (six isolates; three/three), diphtheroids (three isolates; two/one), bacteroides (two isolates; 0/two), S pneumoniae (two isolates; one/one), and enterococcus (one isolate; 0/one). From the above, it appears that S aureus, E coli, and perhaps proteus were the most significant isolates in that they occurred proportionally more often in cases judged to have infection at necropsy.
A retrospective study of septicaemia in premature infants reported the findings in 256 infants with organisms grown from blood cultures.12 This was from a total intake of 2906 infants of birth weight 1001–2500 g admitted to one hospital. There were 172 isolates that were judged to be significant; this was based on a known pathogen isolated from more than one site and associated with the appropriate clinical features. In total, 129 isolates were judged to be contaminants; these were either non‐pathogens, such as diphtheroids, lactobacilli, or α haemolytic streptococci, or pathogens isolated from a single flask and not associated with clinical evidence of infection. Septicaemia was diagnosed in 158 infants on the basis of blood culture results and the mortality rate was 50%. There were 98 infants with contaminated cultures and they had a mortality rate of 13.3%. The mortality rate was 6.6% in the 2650 infants with no culture taken or negative cultures. Staphylococci were isolated from 66 infants with septicaemia. There were 24 coagulase positive staphylococci (S aureus) and these patients had a mortality rate of 38%; the other 42 isolates were coagulase negative (S epidermidis) and the mortality rate was 9%. Gram negative rods (such as E coli, klebsiella, pseudomonas, and proteus) were isolated from 91 patients, with a mortality rate of 65%. There were 79 deaths from septicaemia; significant organisms were isolated from antemortem cultures alone in 15, from both antemortem and postmortem cultures in 26, and from postmortem cultures alone in 38. The calculated sensitivity of postmortem blood cultures for the diagnosis of septicaemia in this series is 81%. The authors reported the contamination rate in blood cultures obtained in life to be 4% (95% confidence interval, 3.2% to 4.8%).
The introduction of tissue procurement centres with “sterile morgue facilities” allowed a new method of evaluating human postmortem microbiology. O'Toole and colleagues reported their findings in 54 necropsy cases in which 440 tissue samples were obtained.13 Cases were selected in which there was no premortem evidence of infection. An elaborate procedure was used to clean the skin of the corpse; the staff undertook a “surgical scrub” and “surgical gowning procedure”; the dissection was carried out in a room with controlled airflow; new sterile instruments were used to obtain each tissue sample. The samples were cultured in a range of media to detect both aerobic and anaerobic organisms. The interval from death to necropsy was under 20 hours. In 25 cases there was no growth in the samples obtained. In 29 cases there was growth in one or more sample, yielding 48 different isolates. Most isolates were considered to be external contaminants, such as diphtheroids, α haemolytic streptococci, and S epidermidis (26 isolates in total). Isolates thought possibly to be significant included E coli (three isolates), S aureus (three isolates), C albicans (two isolates), E aerogenes (two isolates), P aeruginosa (two isolates), enterococcus (one isolate), and Shigella flexneri (one isolate). There was no growth in 324 (74%) of the 440 samples obtained. Growth in the spleen occurred in 12 of 47 samples obtained; in six cases, the growth was judged to be significant because there was evidence at necropsy of infection. In two cases, the organisms were thought to be external or internal contaminants rather than a consequence of agonal spread. In the remaining four cases agonal spread was a possible explanation.
Minckler and colleagues reported their experience with the collection of tissue samples from 262 surgical operations and 213 necropsies.14 The techniques of the “sterile necropsy” are as described in the preceding paragraph. A total of 263 tissue samples were obtained at surgery, but 114 were from the gastrointestinal tract, which has a resident flora, and others came from the skin and lung, which are also likely to be contaminated. There were 109 surgical samples of internal organs and 92 were sterile (84%). The equivalent organs obtained at necropsy totalled 738 and 485 were sterile (66%). There were 23 different organisms isolated, four (E coli, enterobacter, S epidermidis, and S aureus) of which accounted for 53.9%.
Roberts assessed the bacteriological results in 100 necropsies.15 The surface of the relevant organ was sterilised by heat and then heart blood or tissue was obtained. The cultures of heart blood yielded no growth in 63%, in 22% the results were positive and judged to be indicative of genuine infection, and in 15% the results were positive but there was no evidence of infection. The corresponding results for the spleen were no growth in 77%, genuine infection in 19%, and false positives in 4%.
A study of 91 unselected necropsies on individuals aged 7 to 75 years conducted between one and 23 hours after death yielded 39% positive in heart blood cultures and 63% positive in lung cultures.16 Once again, the surface of organs was sterilised by heat before the sample was obtained.
Dolan and colleagues obtained 211 tissue specimens from 67 necropsies.17 The patients ranged from 5 to 88 years (mean, 55.1). The specimens were obtained using non‐sterile instruments and sent to the laboratory where the capsular surfaces were seared by heat and then tissue was taken for culture. Bacteria or fungi were isolated from 58.3% of specimens. The spleen was positive in 23 of 53.
Wise cultured tissue from the spleen in 192 consecutive necropsies on individuals aged 31 to 91 years.18 Twenty four of the 192 cultures (12.5%) yielded a growth of organisms: E coli in 11, Proteus sp. in five, S aureus in two, S epidermidis in one, Streptococcus faecalis in one, P aeruginosa in one, and mixed faecal organisms in three. The mean time from death to necropsy was over 24 hours. The splenic tissue was obtained after heat sterilisation of the splenic surface.
“Postmortem bacterial cultures proved useful in the diagnosis of systemic infection in neonatal deaths in a study by Eisenfield and colleagues”
Pryse‐Davies and Hurley conducted a retrospective review of 835 perinatal necropsies performed between 1967 and 1976 in which there had been careful bacteriological assessment.19 The series comprised 130 aborted fetuses over 500 g, 371 stillborn fetuses, 307 neonates dying in the 1st week of life, and 27 neonates dying later in the 1st month of life. A swab of heart blood was obtained from the right ventricle after heat sterilisation of the surface; swabs were obtained from the bronchi after opening the larynx and trachea with flamed scissors; and CSF was obtained by cisternal puncture after wiping the skin with 70% ethyl alcohol. In total, 479 CSF specimens were examined; 58 were positive (12%) yielding 69 isolates. Twenty one percent of 797 heart blood cultures were positive and 41% of 795 bronchial swabs yielded bacterial growth. There were 114 cases with evidence of disseminated bacterial infection; this was defined as a positive blood culture with the same organism at one or more other sites. The most common organisms were “pseudomonads” (33 isolates), E coli (24 isolates), staphylococci (14 isolates), Klebsiella pneumoniae (14 isolates), streptococci (13 isolates), P aeruginosa (five isolates), and Proteus mirabilis (three isolates). The 69 isolates from CSF included E coli (13 isolates), staphylococci (13 isolates), streptococci (12 isolates), pseudomonads (11 isolates), K pneumoniae (eight isolates), Enterobacter cloacae (three isolates), and C perfringens (two isolates). Varying degrees of histological evidence of meningitis were found in nine cases; in four babies meningitis was considered the primary cause of death. In 47 cases the CSF was positive but there were no histological changes of meningeal inflammation.
Postmortem bacterial cultures proved useful in the diagnosis of systemic infection in neonatal deaths in a study by Eisenfield and colleagues.20 They obtained blood and CSF from 311 infants; in 293 cases the samples were obtained within two hours of death and 148 of these infants had also had blood and/or CSF cultures in life. In 18 infants only antemortem cultures were obtained. The blood cultures were positive in 38% (118 of 311 cases) and CSF cultures were positive in 16% (51 of 311 cases). In 73 cases, Gram negative organisms were isolated, in 32 streptococcal organisms, in 10 S aureus, and in 11 the isolates were mixed. The validity of the postmortem cultures was indicated by:
- Of 44 infants with bacteriological evidence of infection before death, postmortem cultures were also positive, despite antibiotic treatment, in 26. In 25 of the 26 the organism found at necropsy was the same as that found before death.
- In all 43 infants with both bacteraemia and meningitis bacteria isolated from both sites were identical.
Sonnabend and colleagues undertook a careful bacteriological study of 70 babies who suffered from SUDI, aged from 21 to 355 days.21 Eighty percent were examined between two and 24 hours after death. Specimens for microbiology included heart blood, lung, liver, spleen, kidney, brain, and segments of small and large intestine. Eight of the 70 babies had evidence of overwhelming bacterial infection as the cause of death. This was based on the results of postmortem cultures. They commented that: “the postmortem cultures were of diagnostic value, providing the sole means of identifying the cause of death in eight of the 70 infants, in whom the presence of an infection could be established only after repeated and extensive histological investigations”. The causative organisms (nine isolates) were H influenzae type b (two cases), S pneumoniae (two cases), S aureus (two cases), E coli (two cases), and group B streptococcus (one case).
A study of SUDI in Avon, UK, between 1987 and 1989, included bacteriological assessment of throat swabs in patients and a comparison group of healthy age and season matched normal infants.22 Postmortem examinations were conducted on 95 babies affected by SUDI aged 1 week to 2 years. Throat swabs, blood for culture, and CSF were obtained within a median interval of 3.5 hours from the discovery of death (range, 0.25–46). Further tissue samples were obtained when the necropsy was conducted (median time, 25 hours; range, 2.8–73). The blood cultures yielded bacterial pathogens in eight of 95 cases. Spleen cultures were positive for pathogens in four of 94. The CSF was positive for pathogens in one of 95. It is not clear from the report how many cultures contained organisms considered to be contaminants. Comparison of the upper respiratory flora in babies with SUDI and healthy infants showed increased carriage of:
- Staphylococcus aureus; 24 of 95 in cases versus 12 of 190 (6%) in controls; odds ratio (OR), 5 (95% confidence interval (CI), 2.4 to 10).
- Coliforms, 15 of 95 in cases versus two of 190 (1%) in controls; OR, 29 (95% CI, 7.5 to 111).
- Streptococcus pneumoniae, 10 of 95 in cases versus two of 190 (1%) in controls; OR, 10 (95% CI, 2.9 to 34).
- Group B streptococcus, six of 95 in cases versus two of 190 (1%) in controls; OR, 11 (95% CI, 1.9 to 63).
Sadler stresses the value of bacteriological investigations in cases of SUDI.23 He presents data on 95 necropsies of patients aged 3 days to 29 months, 63 of whom presented as “cot deaths”. The protocol included obtaining CSF by cisternal puncture, blood from the subclavian vein, and lung tissue and spleen at necropsy. Nine diagnoses depended on bacteriology results: pneumococcal meningitis and septicaemia (two cases), pneumococcal meningitis (two cases), meningococcal meningitis (two cases), group B streptococcal meningitis and septicaemia, H influenzae pneumonia and septicaemia, and H influenzae meningitis. Sixty one specimens of CSF were cultured: 35 showed no growth, six showed significant growth, and 24 grew organisms considered to be contaminants/commensals. Forty five specimens of blood were cultured and only nine showed no growth, five showed significant growth, and 31 grew contaminants/commensals. Twenty one specimens of spleen were examined, 13 had no growth, none had significant growth, and eight grew contaminants/commensals. The degree of contamination found in these specimens is clearly much higher than in the other studies noted above. The median time between death and necropsy was five hours.
The CESDI‐SUDI study was a case control study of SUDI carried out in England between 1993 and 1996.24 There were 456 cases of SUDI and four controls for each case. All cases of SUDI had a detailed necropsy, which included the examination of specimens for bacteriology. Blood cultures were recorded in 287 cases, and 144 (50%) were sterile. Mixed growth was obtained in 130 cases (45%). A major pathogen with corresponding histological changes was found in seven cases; in eight cases there was a single pathogen without corresponding histology. In the latter group the organisms were S aureus in three cases, group B β haemolytic streptococcus in three cases, and α haemolytic streptococcus in two cases. There were 279 cultures of CSF, 211 (76%) of which were sterile. There were seven positive cultures with corresponding histological changes in the brain. The organisms were N meningitidis (three cases), H influenzae type B, E coli, group A β haemolytic streptococcus, and S epidermidis. In a further 10 patients a single pathogenic organism was grown from CSF without corresponding histological changes in the brain. The organisms listed were N meningitidis, S pneumoniae (two cases), haemolytic streptococcus (three cases), and haemophilus (two cases). In most of the last group, the organisms were grown from other sites and were associated with respiratory tract infection. In 51 other cases there was a mixed growth or single organisms considered to be contaminants. Thus, blood cultures yielded 15 possibly significant results (5.2%) and CSF cultures yielded 17 positives (6%).
Blood–brain barrier
The blood–brain barrier was first defined by Ehrlich and his students.25,26,27 Analine dyes, such as trypan blue, were injected intravenously into experimental animals. The animals were subsequently sacrificed and it was noted at necropsy that the tissues were stained blue, but this did not occur in the brain or CSF. The dyes combined with albumin in the bloodstream and would pass with albumin through the walls of peripheral capillary vessels, but the vessels in the brain formed a barrier. If trypan blue was injected into the CSF then the brain and CSF were stained blue at necropsy, the peripheral tissues were not.
The capillary endothelial cells in the brain have tight junctions, lack fenestrations, have a paucity of pinocytic vesicles, but contain increased numbers of mitochondria.28 The tight junctions exclude large molecules such as albumin (molecular weight, 69 000). There are areas of the hypothalamus, the area postrema, and the subfornical and subcommisural organs where the endothelial cells lack tight junctions. This allows diffusion from the plasma to hypothalamic osmoreceptors and the chemoreceptors of the area postrema and the subfornical and subcommisural regions. However, there is free diffusion of molecules as large as inulin (molecular weight 5000) between the ventricular fluid and brain interstitial fluid.
Molecules can be transported across brain endothelial cells by pinocytosis, which is an active process. This can be studied in experimental animals using horseradish peroxidase, which is then visualised by electron microscopy. Hossmann and Olsson studied the effects of ischaemia on vascular permeability in the cat.29 Evans blue and horseradish peroxidase were injected intravenously and localised by fluorescence and electron microscopy, respectively. Acute complete cerebral ischaemia produced by arterial ligation for 15 minutes to three hours did not lead to extravasation of the tracers. The tight junctions were maintained and transfer of horseradish peroxidase by pinocytosis ceased.
In inflammation, white blood cells cross the endothelial cells by emperipolesis, but large molecules cross by a combination of pinocytosis and or leakage through opening of the tight junctions.27 The result is that the composition of the CSF in meningitis is closer to that of plasma.
Changes in the composition of body fluids, including CSF and vitreous humour, after death have been reviewed by Coe.30 In general, total proteins and the albumin to globulin ratio are in the ranges found in life. Thus, after death the blood–brain barrier is maintained, at least in relation to large molecules. Mangin and colleagues obtained samples of CSF within 24 hours of death (three to 24 hours) in 42 necropsies on patients aged 5 to 74 years.31 The CSF protein was within the normal range in a group of 15 who died suddenly (the agonal period was less than 10 minutes). If death was prolonged over six hours, as in those in intensive care, then the mean CSF protein concentration was raised to 8.3 g/litre. This is presumably a result of circulating cytokines in life causing increased permeability of the brain capillaries.
“After death the blood–brain barrier is maintained, at least in relation to large molecules”
A change that does occur in CSF composition after death is that the white blood cell count rises.32,33 The CSF cell count ranged in adults from 1 to 108 ×106 cells/litre and in cases of sudden infant death syndrome from 37 to 3250 ×106 cells/litre.32 The cell count rises with time after death; the cells are mononuclear and can be typed as lymphocytes or monocytes in the first 12 hours, but thereafter typing and morphological assessment is no longer reliable. Polymorphs are not seen in the absence of inflammation. The postmortem CSF pleocytosis is probably a result of detachment of the meningeal lining cells.
Discussion
Many of the studies described above used stringent precautions to reduce the possibility of contamination, but it cannot be avoided completely. Wise sterilised the surface of the spleen at necropsy before obtaining a specimen and found only 12.5% (95% CI, 7.5% to 17.5%) positive cultures in 192 cases with a long postmortem interval.18 Dolan and colleagues by comparison grew bacteria from 23 of 53 (43%; 95% CI, 26% to 61%) splenic samples.17 The difference was that in Dolan's study the specimens were obtained using non‐sterile instruments and the surfaces were subsequently sterilised in the laboratory. This difference is presumably the result of an increased rate of contamination because there is no reason to suppose an increased rate of infection, agonal change, or postmortem translocation in the second study. Even with blood cultures taken in life and with the most careful techniques contamination is always a potential problem.1,2,34 Buetow and colleagues estimated the contamination rate in over 2000 blood cultures obtained in life as 4% (95% CI, 3.2% to 4.8%).12
Studies in which stringent precautions have been taken to reduce contamination show that most blood cultures are sterile (table 11),), so that agonal invasion of the bloodstream does not occur as a universal phenomenon, at least in numbers sufficient to produce a positive result.
In the largest series reported by Carpenter and Wilkins10 blood cultures were negative in 68% (95% CI, 64% to 72%). Results from most of the other studies are in a similar range, and it appears that in adult necropsy practice around two thirds of blood cultures and splenic cultures will be negative if the specimens are taken with care. Carpenter and Wilkins also found a single isolate in 25% (95% CI, 23% to 28%) and mixed growth in 7% (95% CI, 6% to 8%). If mixed growth is equated with the three postmortem artefacts of agonal spread, postmortem translocation, and contamination—and if contamination at necropsy is similar in magnitude to the 4–6% found in life—then agonal change is a rare event. The study by Wise also indicates that agonal spread is rare because he found mixed growth in only 1.5% of cases.18 Thus, the concept that the entry of several bacterial species at the time of death is inevitable is incorrect. If it occurs at all it is rare. None of the studies deals specifically with the relation between length of resuscitation and isolation rates, but many of the cases in the later studies will have had the agonal phase prolonged for varying intervals by modern resuscitation methods. The rest of the results in the table are broadly in line with the above, although there are exceptions.11 In the series of Carpenter and Wilkins most single isolates were judged to be causing genuine and significant disease.10 A minority could represent bacteraemia not causing disease, and some of these episodes could have arisen close to the time of death. Thus, it is possible that a small proportion of episodes of bacteraemia arise in the agonal period, but this is different to the usual concept of agonal spread.
The postmortem interval has only a small effect on blood culture isolation rates. Some of the authors noted no effect,6,9,19 and some only a small effect,10,11 but only the study by Carpenter and Wilkins had sufficient statistical power to answer the question. The authors found that there was a significant increase in isolation rate with postmortem interval, and the best fit regression line fell from 80% sterility to 60% sterility over 18 hours. This observation does not necessarily support the idea of postmortem translocation because it is possible that a small number of organisms present in the bloodstream at the time of death could continue to grow, so that the chance of a positive culture increases with time.
“The concept that the entry of several bacterial species at the time of death is inevitable is incorrect”
In general, a pure growth of a pathogen in a blood culture is probably associated with genuine infection, but in many of the reports it is not possible to enumerate the relative proportion of true positives and false positives. The report by Fredette is an exception, because he lists the cause of death and the blood culture results for each patient.3 Some of the causes given are unusual according to modern concepts, but this is the era when lobar pneumonia and puerperal fever killed otherwise young healthy people, and there is good correspondence between the results of culture and the anatomical diagnoses. In this study the isolation of S pneumoniae, S pyogenes, and S aureus in general indicate significant infection. It is equally important to note that even when a confident diagnosis of lobar pneumonia was made in the era before antibiotic treatment, S pneumoniae was isolated in less than 50% of cases in blood taken within 30 minutes of death. Clearly, there are false negative and false positive results. Several authors have tried to correlate antemortem and postmortem blood culture results in an attempt to assess the veracity of the latter.3,11,12,15,20 There is a degree of concordance in these studies, but if the results differ it does not necessarily imply that the postmortem result is unreliable; equally, if the results are concordant this does not prove that the postmortem result is correct.
The value of a pathological test is best expressed in terms of sensitivity and specificity. This then allows the NPV and PPV values to be calculated at different levels of a priori probability. We have made calculations where possible of these parameters and they are listed in the historical review. The problem is that there is too much uncertainty concerning the anatomical diagnosis of infection and the number of cases in most studies is too small for reliable estimates. It would be best to calculate sensitivity and specificity for specific bacterial isolates as markers for specific conditions (for example, pneumococcus as a marker for lobar pneumonia), but in general this has not proved possible.
The postmortem bacteriological results in the perinatal and neonatal period are similar to those in adults. Pryse‐Davies and Hurley obtained blood cultures in 797 infants in the perinatal age range.19 There was a single isolate in 17% (95% CI, 14.4% to 20.2%), mixed growth in 4% (95% CI, 2.4% to 5.1%), and sterile cultures in 79% (95% CI, 72.7% to 85.1%). The clinical features were in keeping with the rule that most single isolates of pathogens (> 50%) occurred in association with genuine infection. These results were obtained with standard necropsy practice in which only one third of cases were examined in less than 24 hours. Cultures of CSF yielded mixed growth in 2% (95% CI, 0.9% to 3.6%), a pure culture in 10% (95% CI, 7% to 12.6%), and sterile cultures in 88% (95% CI, 79.5% to 96.3%). Once again, single isolates of known pathogens were concordant with genuine infection. The very low level of mixed isolates in the CSF is probably secondary to contamination, and therefore these results challenge the concept that agonal spread of organisms from the mucosal surface through the blood to the CSF can occur. Eisenfield and colleagues also found a high rate of sterility in CSF samples from 311 neonates.20 Positive cultures were obtained in 51 of 311 cases (16.4%; 95% CI, 11.9% to 20.9%). Evidence suggested that in at least 43 of the 51 positive cases there was genuine CSF infection; thus, the level of postmortem artefact is close to the minimum level for contamination, and this argues against agonal spread to the CSF.
Interpretation of blood and CSF culture results is of particular importance in SUDI and sudden infant death syndrome. It has long been suspected that disseminated or overwhelming infection has a role in the pathogenesis of sudden death in infancy and microbiological investigation is a standard part of the necropsy protocol.24 If the specimens are taken with care, most blood and CSF cultures are negative.8,21 In the few that are positive and indicate genuine infection the diagnosis can often be made with histological examination alone. This has led many workers to become disenchanted with bacteriological investigation and they either neglect to obtain the samples,24 or take them without adequate care. The result is many contaminated cultures and the view has developed that postmortem microbiology is of little value and the results are difficult to interpret. This can be seen by comparing the level of contaminants in Adelson and Kinney's 1956 study,8 with that of Sadler in 1998,23 and the CESDI‐SUDI study published in 2000.24 Adelson and Kinney had no growth in 79% (95% CI, 63.3% to 95%) of 120 blood cultures. The postmortem interval is not stated but is probably over 24 hours in many of the cases. The results indicate that if agonal spread and/or postmortem translocation occur then it can only be in a minority. However, the authors were unsure of the relevance of the positive isolates and this remains the problem in SUDI and sudden infant death syndrome. Is a pure growth of E coli or of S aureus the cause of death, do they indicate bacteraemia unrelated to death, or are they postmortem artefacts? The studies in adults and in neonates suggest that few of the results are postmortem artefacts and most represent episodes of bacteraemia that may or may not contribute to death. The authors had no growth in 38 CSF samples (0%; 95% CI, 0% to 8%). This result also indicates that agonal spread and/or postmortem translocation from the mucosal surface to the CSF probably does not occur.
Sonnabend and colleagues also undertook a careful bacteriological study of SUDI. They report their results in a different way to most of the other investigations and it is not clear how many cultures had contaminants. The authors were prepared to diagnose the cause of death on the basis of the microbiological results alone. Their assessment depended on the nature of the organism grown and the number of positive sites. Eight of 70 patients were judged to have disseminated bacterial infection. Sadler was also prepared to diagnose the cause of death in SUDI on the basis of a significant pathogen isolated at necropsy in the absence of histological confirmation.23
“Interpretation of blood and cerebrospinal fluid culture results is of particular importance in sudden unexplained death in infancy and sudden infant death syndrome”
The study of SUDI by Gilbert et al repays careful analysis.22 The authors found increased throat carriage of S aureus (OR, 5), coliforms (OR, 29), and group B streptococci (OR, 11) in a comparison with normal healthy age matched infants. In epidemiological studies with the requisite statistical power, ORs of this level would normally be judged as possible causative factors. However, in this case, the authors were cautious because of the possibility of postmortem artefact. In simple terms, they did not trust the microbiological results obtained after death because of the possibility that the bacteria had in some way been introduced during the interval between death and obtaining the specimen. A mechanism by which this could occur, however, is not clear. Harrison and colleagues in a later study found a very low isolation rate of coliforms and other Gram negative bacilli in the nasopharynx of normal healthy live infants aged 0–6 months.35 Most of these infants slept supine at this age. In later months, many of the infants slept prone; pernasal swabs obtained in the early morning from infants sleeping prone who had a clinical upper respiratory tract infection often contained coliforms and other Gram negative bacilli. These organisms appeared to grow up overnight in secretions pooling in the upper airways when the infants were lying in the prone position. The bacteria disappeared from swabs obtained later in the day when the infants were up and awake and had swallowed the secretions. Thus, the association between coliforms in the upper airways and SUDI is not a postmortem artefact but could be a premortem artefact. The comparison in the study by Gilbert et al was in fact between the early morning upper respiratory tract flora of infants who had been sleeping prone (prone sleeping was prevalent at the time of the study) and had an increased incidence of clinical upper respiratory tract infection compared with normal infants later in the day who had a lower incidence of clinical upper respiratory tract infection. One cannot conclude that the bacterial isolates from the throat swabs are causally linked to SUDI, but ORs of 5, 11, and 29 are usually taken seriously, and they should be regarded as at least candidate organisms for a causal role. The association is biologically plausible because some of the species isolated can produce potent toxins (for example, pyogenic toxins of S aureus and soluble toxins of E coli) that could contribute to death by means similar to those observed in toxic shock syndrome.
There is a barrier between the blood and the brain preventing the egress of large molecules, such as albumin, and red and white blood cells. The same barrier reduces the chance of circulating bacteria entering the CSF and brain tissue. Thus, even if there is agonal invasion of the body by bacteria, there is a further barrier to invasion of the brain, and in practice it would appear that CSF cultures are reliable if contamination can be avoided. The chance that bacterial isolates are the result of agonal spread or postmortem translocation is low. The examination of CSF for bacterial infection in life includes not only culture but also differential cell counts and the measurement of total protein.27 The cell count alone is not useful in postmortem CSF samples because mononuclear cells occur normally in the CSF after death,30,31 but polymorphs indicate inflammation and should be counted. The problem is that the half life of polymorphs in the CSF is short and unless the postmortem interval is also short they are unlikely to be seen. The measurement of CSF protein might help in interpretation because there is evidence that the blood–brain barrier is maintained for up to 24 hours after death.31
Our analysis shows that if postmortem cultures are taken with care, most specimens of blood and CSF will be negative, and a substantial proportion of those that are positive will be the result of genuine infection. This applies in the perinatal period, in infancy, and in adults. Establishing that the isolate represents genuine infection is not easy. One useful rule is that a pure culture of a pathogen points to infection, whereas a mixed culture of non‐pathogens is more likely to be some form of postmortem artefact. This rule is useful, but is not an absolute indicator, and more corroborative evidence is required. Examination of a CSF specimen taken soon after death would be one form of corroboration, as indicated above. In life, bacteria are cleared from the bloodstream by a variety of processes, including phagocytosis, and this can lead to release of bacterial DNA and RNA. The polymerase chain reaction could then be used to amplify bacterial ribosomal RNA in blood and CSF. Genuine infection, in theory, is more likely to be accompanied by free bacterial specific RNA than is growth secondary to postmortem artefact.36 The potential of this methodology should be investigated.
Conclusions
The concept that in the agonal period bacteria of several different species are liable to spread from the mucosal surface into the bloodstream producing mixed cultures is incorrect. Agonal spread in this sense into the blood is rare and into the CSF is very rare. The possibility that a single bacterial species invades in the agonal period is not excluded, but this will depend on factors relating to that organism, such as propensity to invade and density of colonisation, or to local factors affecting its site of colonisation, but not to a general diminution of body defence or a general impairment of mucosal integrity.
Postmortem translocation is only a minor problem if samples are obtained within 24 hours of death, or if the body is maintained at 4°C before necropsy.
The major problem with postmortem samples is contamination, but this can be reduced to levels similar to those for samples obtained in life if stringent precautions are taken.
The importance of a single isolate in blood or in CSF should be analysed as it would be in life. In most cases, a pure growth of a recognised pathogen will be associated with genuine infection confirmed from the clinical picture and by histological changes in tissues. However, the presence of a pathogen in the blood does not necessarily indicate significant disease—this is true in life and presumably also applies in death—but if an episode of bacteraemia occurs before death in a patient in whom the cause of death is otherwise unascertained then the organism should be regarded as at least a possible contributing factor.
Take home messages
- Postmortem bacterial cultures can be useful in assessing premortem bacterial invasion
- The main postmortem artefact is contamination, but this can be considerably reduced by careful technique
- Agonal spread appears to be less common than is normally assumed
- Postmortem translocation is not a problem if the body is appropriately stored
- A pure growth of a pathogen in blood or cerebrospinal fluid should be regarded as a possible contributing factor to death at all ages, but corroborative evidence should be sought using a range of techniques
Confirmation of the significance of an isolate should be sought. This involves the analysis of the clinical history and examination of tissues for evidence of inflammation. In addition, CSF samples should be examined—as in life—with cell count, differential, and protein estimation; however, this will only be of value if the sample is obtained soon after death. Modern molecular techniques should also be used to aid diagnosis. If a bacterial isolate has caused significant disease there will probably be dead bacteria and live bacteria in the circulation, and this can be ascertained by amplifying bacteria specific ribosomal RNA using the polymerase chain reaction.
“Postmortem microbiology has most to offer when death is otherwise unascertained, but interpretation is at its most difficult in these cases”
Our literature review has been mainly concerned with systemic invasion by bacteria, but the principle that postmortem samples are useful and should be analysed also applies to postmortem mucosal samples. The mucosal flora at necropsy can be compared with community controls and the publication by Gilbert et al indicates how useful that approach can be.22
Postmortem microbiology has most to offer when death is otherwise unascertained, but interpretation is at its most difficult in these cases. SUDI is a particular problem in that if death is rapid inflammation might not be apparent to corroborate a bacterial isolate. In these cases, samples need to be taken as soon after death as possible and before necropsy. They should be obtained with a full aseptic technique, as in life. The CSF should be submitted for urgent examination as it would be in life. Modern molecular techniques should be used to look for bacterial toxins in blood and CSF and the polymerase chain reaction should be used to detect bacteria specific ribosomal RNA.
Abbreviations
CI - confidence interval
CSF - cerebrospinal fluid
NPV - negative predictive value
OR - odds ratio
PPV - positive predictive value
SUDI - sudden unexpected death in infancy
References
1. Weinbaum F I, Lavie S, Danek M. et al Doing it right the first time: quality improvement and the contaminant blood culture. J Clin Microbiol 199735563–565.565 [PMC free article] [PubMed]
2. Weinstein M P. Blood culture contamination: persisting problems and partial progress. J Clin Microbiol 2003412275–2278.2278 [PMC free article] [PubMed]
3. Fredette J W. Bacteraemia in the agonal period. J Lab Clin Med 19162180–188.188
4. Giordano A S, Barnes A R. Studies in postmortem bacteriology: value and importance of cultures made postmortem. J Lab Clin Med 19227538–546.546
5. Hunt H F, Barrow E, Thompson et al A bacteriological study of 567 post‐mortem examinations. J Lab Clin Med 192914907–912.912
6. Burn C G. Postmortem bacteriology. J Infect Dis 193454395–403.403
7. Burn C G. Experimental studies of postmortem bacterial invasion in animals. J Infect Dis 193354388–394.394
8. Adelson L, Kinney E R. Sudden and unexpected death in infancy and childhood. Pediatrics 195617663–699.699 [PubMed]
9. Kurtin J J. Studies in autopsy bacteriology. Am J Clin Pathol 195830239–243.243 [PubMed]
10. Carpenter H M, Wilkins R M. Autopsy bacteriology: review of 2033 cases. Arch Pathol 19647773–81.81 [PubMed]
11. Wood W H, Oldstone M, Schultz R B. A re‐evaluation of blood culture as an autopsy procedure. Am J Clin Pathol 196543241–247.247 [PubMed]
12. Buetow K C, Klein S W, Lane R B. Septicaemia in premature infants. The characteristics, treatment and prevention of septicaemia in premature infants. Am J Dis Child 196511029–41.41 [PubMed]
13. O'Toole W F, Saxena H M, Golden A. et al Studies of postmortem microbiology using sterile autopsy technique. Arch Pathol 196580540–547.547 [PubMed]
14. Minckler T M, Newell G R, O'Toole W F. et al Microbiology experience in collection of human tissue. Am J Clin Pathol 19664585–92.92 [PubMed]
15. Roberts F J. A review of postmortem bacteriological cultures. Can Med Assoc J 196910070–74.74 [PMC free article] [PubMed]
16. Koneman E W, Minckler T M, Shires D B. et al Postmortem microbiology II. Selection of cases for culture. Am J Clin Pathol 19715517–23.23 [PubMed]
17. Dolan C T, Brown A L, Ritts R E. Microbiological examination of postmortem tissues. Arch Pathol 197192206–211.211 [PubMed]
18. Wise R. The “septic spleen”—a critical evaluation. J Clin Pathol 197629228–230.230 [PMC free article] [PubMed]
19. Pryse‐Davies J, Hurley R. Infections and perinatal mortality. J Antimicrob Chemother 19795(suppl A)59–70.70 [PubMed]
20. Eisenfield L, Ermocilla R, Wirtschafter D. et al Systemic bacterial infections in neonatal deaths. Am J Dis Child 1983137645–649.649 [PubMed]
21. Sonnabend O A, Sonnabend W F, Krech U. et al Continuous microbiological and pathological study of 70 sudden and unexpected infant deaths: toxigenic intestinal Clostridium botulinum infection in 9 cases of sudden infant death syndrome. Lancet 19851237–240.240 [PubMed]
22. Gilbert R, Rudd P, Berry P J. et al Combined effect of infection and heavy wrapping on the risk of sudden unexpected infant death. Arch Dis Child 199267171–177.177 [PMC free article] [PubMed]
23. Sadler D W. The value of a thorough protocol in the investigation of sudden infant deaths. J Clin Pathol 199851689–694.694 [PMC free article] [PubMed]
24. Fleming P, Bacon C, Blair P. et al Sudden unexpected deaths in infancy. In: The CESDI‐SUDI studies 1993–1996. London: The Stationery Office, 200097–112.112
25. Ehrlich P. Das Sauerstoffbeduerfnis des Organismus. Eine farbenanalytische Studie. Berlin: A Hirschfeld, 1885
26. Friedmann U. Blood–brain barrier. Physiol Rev 194222125–145.145
27. Fishman R A. Blood–brain barrier. In: Cerebrospinal fluid in diseases of the nervous system. London: WB Saunders Company, 198043–69.69
28. Oldendorf W H, Cornford M E, Brown W J. The large apparent work capability of the blood–brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol 19771409–417.417 [PubMed]
29. Hossmann K A, Olsson Y. The effect of transient cerebral ischaemia on the vascular permeability to protein tracers. Acta Neuropathol 197118103–112.112 [PubMed]
30. Coe J I. Post‐mortem chemistry update. Emphasis on forensic applications. Am J Forensic Med Pathol 19931491–117.117 [PubMed]
31. Mangin P, Lugnier A A, Chaumont A J. et al Forensic significance of postmortem estimation of the blood cerebrospinal fluid barrier permeability. Forensic Sci Int 198322143–149.149 [PubMed]
32. Platt M S, McClure S, Clarke R. et al Post‐mortem cerebrospinal fluid pleocytosis. Am J Forensic Med Pathol 198910209–212.212 [PubMed]
33. Wyler D, Marty W, Bar W. Correlation between the post‐mortem cell content of cerebrospinal fluid and time of death. Int J Legal Med 1994106194–199.199 [PubMed]
34. Libman E. On some experience with blood cultures in the study of bacterial infections. John Hopkins Hospital Bulletin 190617215–218.218
35. Harrison L M, Morris J A, Telford D R. et al The nasopharyngeal bacterial flora in infancy: effect of age, season, viral upper respiratory infection and sleeping position. FEMS Immunol Med Microbiol 19992519–28.28 [PubMed]
36. Peters R P, van Agtmael M A, Danner S A. et al New developments in the diagnosis of blood stream infections. Lancet Infect Dis 20044751–760.760 [PubMed]
Articles from Journal of Clinical Pathology are provided here courtesy of BMJ Group
Formats:
- Article |
- PubReader |
- ePub (beta) |
- PDF (235K) |
- Citation
- Post-mortem interval and bacteriological culture yield in sudden unexpected death in infancy (SUDI).[Forensic Sci Int. 2010]Weber MA, Hartley JC, Brooke I, Lock PE, Klein NJ, Malone M, Sebire NJ. Forensic Sci Int. 2010 May 20; 198(1-3):121-5. Epub 2010 Mar 11.
- Microbiological findings in sudden unexpected death in infancy: comparison of immediate postmortem sampling in casualty departments and at autopsy.[J Clin Pathol. 2011]Pryce JW, Roberts SE, Weber MA, Klein NJ, Ashworth MT, Sebire NJ. J Clin Pathol. 2011 May; 64(5):421-5. Epub 2011 Mar 8.
- Postmortem bacteriology in forensic pathology: diagnostic value and interpretation.[Leg Med (Tokyo). 2001]Tsokos M, Püschel K. Leg Med (Tokyo). 2001 Mar; 3(1):15-22.
- Interpreting results of ethanol analysis in postmortem specimens: a review of the literature.[Forensic Sci Int. 2007]Kugelberg FC, Jones AW. Forensic Sci Int. 2007 Jan 5; 165(1):10-29. Epub 2006 Jun 19.
- Novel hypothesis for unexplained sudden unexpected death in infancy (SUDI).[Arch Dis Child. 2009]Highet AR, Berry AM, Goldwater PN. Arch Dis Child. 2009 Nov; 94(11):841-3. Epub 2009 May 3.
- Exploring the Risk Factors for Sudden Infant Deaths and Their Role in Inflammatory Responses to Infection[Frontiers in Immunology. 1/01]Blackwell C, Moscovis S, Hall S, Burns C, Scott RJ. Frontiers in Immunology. 1/01/01 00:00; 644
- Ancient human microbiomes[Journal of human evolution. 2015]Warinner C, Speller C, Collins MJ, Lewis CM Jr. Journal of human evolution. 2015/02/01 00:00; 0125-136
- Residual Antibiotics in Decontaminated Human Cardiovascular Tissues Intended for Transplantation and Risk of Falsely Negative Microbiological Analyses[PLoS ONE. 1/01]Buzzi M, Guarino A, Gatto C, Manara S, Dainese L, Polvani G, Tóthová JD. PLoS ONE. 1/01/01 00:00; 9(11)e112679
- Comprehensive Postmortem Analyses of Intestinal Microbiota Changes and Bacterial Translocation in Human Flora Associated Mice[PLoS ONE. 1/01]Heimesaat MM, Boelke S, Fischer A, Haag LM, Loddenkemper C, Kühl AA, Göbel UB, Bereswill S. PLoS ONE. 1/01/01 00:00; 7(7)e40758
- Invasive Group A Streptococcal Infection and Vaccine Implications, Auckland, New Zealand[Emerging Infectious Diseases. ...]Safar A, Lennon D, Stewart J, Trenholme A, Drinkovic D, Peat B, Taylor S, Read K, Roberts S, Voss L. Emerging Infectious Diseases. 2011/06/01 00:00; 17(6)983-989
- Postmortem bacteriology: a re‐evaluationPostmortem bacteriology: a re‐evaluationJournal of Clinical Pathology. 2006 Jan; 59(1)1
- Septic Shock Due to Candidemia: Outcomes and Predictors of Shock DevelopmentSeptic Shock Due to Candidemia: Outcomes and Predictors of Shock DevelopmentJournal of Clinical Medicine Research. 2011 Apr; 3(2)65
- Septic shock attributed to Candida infection: importance of empiric therapy and ...Septic shock attributed to Candida infection: importance of empiric therapy and source control.Clin Infect Dis. 2012 Jun ;54(12):1739-46. doi: 10.1093/cid/cis305. Epub 2012 Mar 15 .PubMed
- Chiropractic Management of Pubic Symphysis Shear Dysfunction in a Patient With O...Chiropractic Management of Pubic Symphysis Shear Dysfunction in a Patient With Overactive BladderJournal of Chiropractic Medicine. 2014 Jun; 13(2)81
- Cellular and Molecular Biology of Candida albicans Estrogen Response Cellular and Molecular Biology of Candida albicans Estrogen ResponseEukaryotic Cell. 2006 Jan; 5(1)180
Your browsing activity is empty.
Activity recording is turned off.
See more...- Doing it right the first time: quality improvement and the contaminant blood culture.[J Clin Microbiol. 1997]Weinbaum FI, Lavie S, Danek M, Sixsmith D, Heinrich GF, Mills SSJ Clin Microbiol. 1997 Mar; 35(3):563-5.
- Review Blood culture contamination: persisting problems and partial progress.[J Clin Microbiol. 2003]Weinstein MPJ Clin Microbiol. 2003 Jun; 41(6):2275-8.
- Review Blood culture contamination: persisting problems and partial progress.[J Clin Microbiol. 2003]Weinstein MPJ Clin Microbiol. 2003 Jun; 41(6):2275-8.
- Doing it right the first time: quality improvement and the contaminant blood culture.[J Clin Microbiol. 1997]Weinbaum FI, Lavie S, Danek M, Sixsmith D, Heinrich GF, Mills SSJ Clin Microbiol. 1997 Mar; 35(3):563-5.
- Sudden and unexpected death in infancy and childhood.[Pediatrics. 1956]ADELSON L, KINNEY ERPediatrics. 1956 May; 17(5):663-99.
- Studies in autopsy bacteriology.[Am J Clin Pathol. 1958]KURTIN JJAm J Clin Pathol. 1958 Sep; 30(3):239-43.
- AUTOPSY BACTERIOLOGY: REVIEW OF 2,033 CASES.[Arch Pathol. 1964]CARPENTER HM, WILKINS RMArch Pathol. 1964 Jan; 77():73-81.
- A RE-EVALUATION OF BLOOD CULTURE AS AN AUTOPSY PROCEDURE.[Am J Clin Pathol. 1965]WOOD WH, OLDSTONE M, SCHULTZ RBAm J Clin Pathol. 1965 Mar; 43():241-7.
- Review SEPTICEMIA IN PREMATURE INFANTS. THE CHARACTERISTICS, TREATMENT, AND PREVENTION OF SEPTICEMIA IN PREMATURE INFANTS.[Am J Dis Child. 1965]BUETOW KC, KLEIN SW, LANE RBAm J Dis Child. 1965 Jul; 110():29-41.
- Studies of postmortem microbiology using sterile autopsy technique.[Arch Pathol. 1965]O'Toole WF, Saxena HM, Golden A, Ritts REArch Pathol. 1965 Nov; 80(5):540-7.
- Microbiology experience in collection of human tissue.[Am J Clin Pathol. 1966]Minckler TM, Newell GR, O'Toole WF, Niwayama G, Levine PHAm J Clin Pathol. 1966 Jan; 45(1):85-92.
- A review of postmortem bacteriological cultures.[Can Med Assoc J. 1969]Roberts FJCan Med Assoc J. 1969 Jan 11; 100(2):70-4.
- Postmortem bacteriology. II. Selection of cases for culture.[Am J Clin Pathol. 1971]Koneman EW, Minckler TM, Shires DB, De Jongh DSAm J Clin Pathol. 1971 Jan; 55(1):17-23.
- Microbiological examination of postmortem tissues.[Arch Pathol. 1971]Dolan CT, Brown AL Jr, Ritts RE JrArch Pathol. 1971 Sep; 92(3):206-11.
- The 'septic spleen'--a critical evaluation.[J Clin Pathol. 1976]Wise RJ Clin Pathol. 1976 Mar; 29(3):228-30.
- Infections and perinatal mortality.[J Antimicrob Chemother. 1979]Pryse-Davies J, Hurley RJ Antimicrob Chemother. 1979 May; 5 Suppl A():59-70.
- Systemic bacterial infections in neonatal deaths.[Am J Dis Child. 1983]Eisenfeld L, Ermocilla R, Wirtschafter D, Cassady GAm J Dis Child. 1983 Jul; 137(7):645-9.
- Continuous microbiological and pathological study of 70 sudden and unexpected infant deaths: toxigenic intestinal clostridium botulinum infection in 9 cases of sudden infant death syndrome.[Lancet. 1985]Sonnabend OA, Sonnabend WF, Krech U, Molz G, Sigrist TLancet. 1985 Feb 2; 1(8423):237-41.
- Combined effect of infection and heavy wrapping on the risk of sudden unexpected infant death.[Arch Dis Child. 1992]Gilbert R, Rudd P, Berry PJ, Fleming PJ, Hall E, White DG, Oreffo VO, James P, Evans JAArch Dis Child. 1992 Feb; 67(2):171-7.
- The value of a thorough protocol in the investigation of sudden infant deaths.[J Clin Pathol. 1998]Sadler DWJ Clin Pathol. 1998 Sep; 51(9):689-94.
- The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat.[Ann Neurol. 1977]Oldendorf WH, Cornford ME, Brown WJAnn Neurol. 1977 May; 1(5):409-17.
- The effect of transient cerebral ischemia on the vascular permeability to protein tracers.[Acta Neuropathol. 1971]Hossmann KA, Olsson YActa Neuropathol. 1971; 18(2):103-12.
- Review Postmortem chemistry update. Emphasis on forensic application.[Am J Forensic Med Pathol. 1993]Coe JIAm J Forensic Med Pathol. 1993 Jun; 14(2):91-117.
- Forensic significance of postmortem estimation of the blood cerebrospinal fluid barrier permeability.[Forensic Sci Int. 1983]Mangin P, Lugnier AA, Chaumont AJ, Offner M, Grucker MForensic Sci Int. 1983 Aug-Sep; 22(2-3):143-9.
- Postmortem cerebrospinal fluid pleocytosis.[Am J Forensic Med Pathol. 1989]Platt MS, McClure S, Clarke R, Spitz WU, Cox WAm J Forensic Med Pathol. 1989 Sep; 10(3):209-12.
- Correlation between the post-mortem cell content of cerebrospinal fluid and time of death.[Int J Legal Med. 1994]Wyler D, Marty W, Bär WInt J Legal Med. 1994; 106(4):194-9.
- The 'septic spleen'--a critical evaluation.[J Clin Pathol. 1976]Wise RJ Clin Pathol. 1976 Mar; 29(3):228-30.
- Microbiological examination of postmortem tissues.[Arch Pathol. 1971]Dolan CT, Brown AL Jr, Ritts RE JrArch Pathol. 1971 Sep; 92(3):206-11.
- Doing it right the first time: quality improvement and the contaminant blood culture.[J Clin Microbiol. 1997]Weinbaum FI, Lavie S, Danek M, Sixsmith D, Heinrich GF, Mills SSJ Clin Microbiol. 1997 Mar; 35(3):563-5.
- Review Blood culture contamination: persisting problems and partial progress.[J Clin Microbiol. 2003]Weinstein MPJ Clin Microbiol. 2003 Jun; 41(6):2275-8.
- Review SEPTICEMIA IN PREMATURE INFANTS. THE CHARACTERISTICS, TREATMENT, AND PREVENTION OF SEPTICEMIA IN PREMATURE INFANTS.[Am J Dis Child. 1965]BUETOW KC, KLEIN SW, LANE RBAm J Dis Child. 1965 Jul; 110():29-41.
- AUTOPSY BACTERIOLOGY: REVIEW OF 2,033 CASES.[Arch Pathol. 1964]CARPENTER HM, WILKINS RMArch Pathol. 1964 Jan; 77():73-81.
- The 'septic spleen'--a critical evaluation.[J Clin Pathol. 1976]Wise RJ Clin Pathol. 1976 Mar; 29(3):228-30.
- A RE-EVALUATION OF BLOOD CULTURE AS AN AUTOPSY PROCEDURE.[Am J Clin Pathol. 1965]WOOD WH, OLDSTONE M, SCHULTZ RBAm J Clin Pathol. 1965 Mar; 43():241-7.
- Studies in autopsy bacteriology.[Am J Clin Pathol. 1958]KURTIN JJAm J Clin Pathol. 1958 Sep; 30(3):239-43.
- Infections and perinatal mortality.[J Antimicrob Chemother. 1979]Pryse-Davies J, Hurley RJ Antimicrob Chemother. 1979 May; 5 Suppl A():59-70.
- AUTOPSY BACTERIOLOGY: REVIEW OF 2,033 CASES.[Arch Pathol. 1964]CARPENTER HM, WILKINS RMArch Pathol. 1964 Jan; 77():73-81.
- A RE-EVALUATION OF BLOOD CULTURE AS AN AUTOPSY PROCEDURE.[Am J Clin Pathol. 1965]WOOD WH, OLDSTONE M, SCHULTZ RBAm J Clin Pathol. 1965 Mar; 43():241-7.
- A RE-EVALUATION OF BLOOD CULTURE AS AN AUTOPSY PROCEDURE.[Am J Clin Pathol. 1965]WOOD WH, OLDSTONE M, SCHULTZ RBAm J Clin Pathol. 1965 Mar; 43():241-7.
- Review SEPTICEMIA IN PREMATURE INFANTS. THE CHARACTERISTICS, TREATMENT, AND PREVENTION OF SEPTICEMIA IN PREMATURE INFANTS.[Am J Dis Child. 1965]BUETOW KC, KLEIN SW, LANE RBAm J Dis Child. 1965 Jul; 110():29-41.
- A review of postmortem bacteriological cultures.[Can Med Assoc J. 1969]Roberts FJCan Med Assoc J. 1969 Jan 11; 100(2):70-4.
- Systemic bacterial infections in neonatal deaths.[Am J Dis Child. 1983]Eisenfeld L, Ermocilla R, Wirtschafter D, Cassady GAm J Dis Child. 1983 Jul; 137(7):645-9.
- Infections and perinatal mortality.[J Antimicrob Chemother. 1979]Pryse-Davies J, Hurley RJ Antimicrob Chemother. 1979 May; 5 Suppl A():59-70.
- Systemic bacterial infections in neonatal deaths.[Am J Dis Child. 1983]Eisenfeld L, Ermocilla R, Wirtschafter D, Cassady GAm J Dis Child. 1983 Jul; 137(7):645-9.
- Sudden and unexpected death in infancy and childhood.[Pediatrics. 1956]ADELSON L, KINNEY ERPediatrics. 1956 May; 17(5):663-99.
- Continuous microbiological and pathological study of 70 sudden and unexpected infant deaths: toxigenic intestinal clostridium botulinum infection in 9 cases of sudden infant death syndrome.[Lancet. 1985]Sonnabend OA, Sonnabend WF, Krech U, Molz G, Sigrist TLancet. 1985 Feb 2; 1(8423):237-41.
- The value of a thorough protocol in the investigation of sudden infant deaths.[J Clin Pathol. 1998]Sadler DWJ Clin Pathol. 1998 Sep; 51(9):689-94.
- The value of a thorough protocol in the investigation of sudden infant deaths.[J Clin Pathol. 1998]Sadler DWJ Clin Pathol. 1998 Sep; 51(9):689-94.
- Combined effect of infection and heavy wrapping on the risk of sudden unexpected infant death.[Arch Dis Child. 1992]Gilbert R, Rudd P, Berry PJ, Fleming PJ, Hall E, White DG, Oreffo VO, James P, Evans JAArch Dis Child. 1992 Feb; 67(2):171-7.
- The nasopharyngeal bacterial flora in infancy: effects of age, gender, season, viral upper respiratory tract infection and sleeping position.[FEMS Immunol Med Microbiol. 1999]Harrison LM, Morris JA, Telford DR, Brown SM, Jones KFEMS Immunol Med Microbiol. 1999 Aug 1; 25(1-2):19-28.
- Review Postmortem chemistry update. Emphasis on forensic application.[Am J Forensic Med Pathol. 1993]Coe JIAm J Forensic Med Pathol. 1993 Jun; 14(2):91-117.
- Forensic significance of postmortem estimation of the blood cerebrospinal fluid barrier permeability.[Forensic Sci Int. 1983]Mangin P, Lugnier AA, Chaumont AJ, Offner M, Grucker MForensic Sci Int. 1983 Aug-Sep; 22(2-3):143-9.
- Review New developments in the diagnosis of bloodstream infections.[Lancet Infect Dis. 2004]Peters RP, van Agtmael MA, Danner SA, Savelkoul PH, Vandenbroucke-Grauls CMLancet Infect Dis. 2004 Dec; 4(12):751-60.
- Combined effect of infection and heavy wrapping on the risk of sudden unexpected infant death.[Arch Dis Child. 1992]Gilbert R, Rudd P, Berry PJ, Fleming PJ, Hall E, White DG, Oreffo VO, James P, Evans JAArch Dis Child. 1992 Feb; 67(2):171-7.
Doing it right the first time: quality improvement and the contaminant blood culture.
Weinbaum FI, Lavie S, Danek M, Sixsmith D, Heinrich GF, Mills SS
J Clin Microbiol. 1997 Mar; 35(3):563-5.
[PubMed] [Ref list]
Review Blood culture contamination: persisting problems and partial progress.
Weinstein MP
J Clin Microbiol. 2003 Jun; 41(6):2275-8.
[PubMed] [Ref list]
3. Fredette J W. Bacteraemia in the agonal period. J Lab Clin Med 19162180–188.188 [Ref list]
4. Giordano A S, Barnes A R. Studies in postmortem bacteriology: value and importance of cultures made postmortem. J Lab Clin Med 19227538–546.546 [Ref list]
5. Hunt H F, Barrow E, Thompson et al A bacteriological study of 567 post‐mortem examinations. J Lab Clin Med 192914907–912.912 [Ref list]
6. Burn C G. Postmortem bacteriology. J Infect Dis 193454395–403.403 [Ref list]
7. Burn C G. Experimental studies of postmortem bacterial invasion in animals. J Infect Dis 193354388–394.394 [Ref list]
Sudden and unexpected death in infancy and childhood.
ADELSON L, KINNEY ER
Pediatrics. 1956 May; 17(5):663-99.
[PubMed] [Ref list]
Studies in autopsy bacteriology.
KURTIN JJ
Am J Clin Pathol. 1958 Sep; 30(3):239-43.
[PubMed] [Ref list]
AUTOPSY BACTERIOLOGY: REVIEW OF 2,033 CASES.
CARPENTER HM, WILKINS RM
Arch Pathol. 1964 Jan; 77():73-81.
[PubMed] [Ref list]
A RE-EVALUATION OF BLOOD CULTURE AS AN AUTOPSY PROCEDURE.
WOOD WH, OLDSTONE M, SCHULTZ RB
Am J Clin Pathol. 1965 Mar; 43():241-7.
[PubMed] [Ref list]
Review SEPTICEMIA IN PREMATURE INFANTS. THE CHARACTERISTICS, TREATMENT, AND PREVENTION OF SEPTICEMIA IN PREMATURE INFANTS.
BUETOW KC, KLEIN SW, LANE RB
Am J Dis Child. 1965 Jul; 110():29-41.
[PubMed] [Ref list]
Studies of postmortem microbiology using sterile autopsy technique.
O'Toole WF, Saxena HM, Golden A, Ritts RE
Arch Pathol. 1965 Nov; 80(5):540-7.
[PubMed] [Ref list]
Microbiology experience in collection of human tissue.
Minckler TM, Newell GR, O'Toole WF, Niwayama G, Levine PH
Am J Clin Pathol. 1966 Jan; 45(1):85-92.
[PubMed] [Ref list]
A review of postmortem bacteriological cultures.
Roberts FJ
Can Med Assoc J. 1969 Jan 11; 100(2):70-4.
[PubMed] [Ref list]
Postmortem bacteriology. II. Selection of cases for culture.
Koneman EW, Minckler TM, Shires DB, De Jongh DS
Am J Clin Pathol. 1971 Jan; 55(1):17-23.
[PubMed] [Ref list]
Microbiological examination of postmortem tissues.
Dolan CT, Brown AL Jr, Ritts RE Jr
Arch Pathol. 1971 Sep; 92(3):206-11.
[PubMed] [Ref list]
The 'septic spleen'--a critical evaluation.
Wise R
J Clin Pathol. 1976 Mar; 29(3):228-30.
[PubMed] [Ref list]
Infections and perinatal mortality.
Pryse-Davies J, Hurley R
J Antimicrob Chemother. 1979 May; 5 Suppl A():59-70.
[PubMed] [Ref list]
Systemic bacterial infections in neonatal deaths.
Eisenfeld L, Ermocilla R, Wirtschafter D, Cassady G
Am J Dis Child. 1983 Jul; 137(7):645-9.
[PubMed] [Ref list]
Continuous microbiological and pathological study of 70 sudden and unexpected infant deaths: toxigenic intestinal clostridium botulinum infection in 9 cases of sudden infant death syndrome.
Sonnabend OA, Sonnabend WF, Krech U, Molz G, Sigrist T
Lancet. 1985 Feb 2; 1(8423):237-41.
[PubMed] [Ref list]
Combined effect of infection and heavy wrapping on the risk of sudden unexpected infant death.
Gilbert R, Rudd P, Berry PJ, Fleming PJ, Hall E, White DG, Oreffo VO, James P, Evans JA
Arch Dis Child. 1992 Feb; 67(2):171-7.
[PubMed] [Ref list]
The value of a thorough protocol in the investigation of sudden infant deaths.
Sadler DW
J Clin Pathol. 1998 Sep; 51(9):689-94.
[PubMed] [Ref list]
24. Fleming P, Bacon C, Blair P. et al Sudden unexpected deaths in infancy. In: The CESDI‐SUDI studies 1993–1996. London: The Stationery Office, 200097–112.112 [Ref list]
25. Ehrlich P. Das Sauerstoffbeduerfnis des Organismus. Eine farbenanalytische Studie. Berlin: A Hirschfeld, 1885 [Ref list]
26. Friedmann U. Blood–brain barrier. Physiol Rev 194222125–145.145 [Ref list]
27. Fishman R A. Blood–brain barrier. In: Cerebrospinal fluid in diseases of the nervous system. London: WB Saunders Company, 198043–69.69 [Ref list]
The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat.
Oldendorf WH, Cornford ME, Brown WJ
Ann Neurol. 1977 May; 1(5):409-17.
[PubMed] [Ref list]
The effect of transient cerebral ischemia on the vascular permeability to protein tracers.
Hossmann KA, Olsson Y
Acta Neuropathol. 1971; 18(2):103-12.
[PubMed] [Ref list]
Review Postmortem chemistry update. Emphasis on forensic application.
Coe JI
Am J Forensic Med Pathol. 1993 Jun; 14(2):91-117.
[PubMed] [Ref list]
Forensic significance of postmortem estimation of the blood cerebrospinal fluid barrier permeability.
Mangin P, Lugnier AA, Chaumont AJ, Offner M, Grucker M
Forensic Sci Int. 1983 Aug-Sep; 22(2-3):143-9.
[PubMed] [Ref list]
Postmortem cerebrospinal fluid pleocytosis.
Platt MS, McClure S, Clarke R, Spitz WU, Cox W
Am J Forensic Med Pathol. 1989 Sep; 10(3):209-12.
[PubMed] [Ref list]
Correlation between the post-mortem cell content of cerebrospinal fluid and time of death.
Wyler D, Marty W, Bär W
Int J Legal Med. 1994; 106(4):194-9.
[PubMed] [Ref list]
34. Libman E. On some experience with blood cultures in the study of bacterial infections. John Hopkins Hospital Bulletin 190617215–218.218 [Ref list]
You are here: NCBI > Literature > PubMed Central (PMC)
Write to the Help Desk Simple NCBI Directory
Subscribe to:
Comments (Atom)

 Facebook
Facebook  Twitter
Twitter  Google+
Google+