| Cranial nerves |
|---|
|
Contents
[hide]Structure[edit]
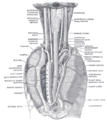
Upon leaving the medulla oblongata between the pyramid and the inferior cerebellar peduncle, the vagus nerve extends through the jugular foramen, then passes into the carotid sheath between the internal carotid artery and the internal jugular vein down to the neck, chest and abdomen, where it contributes to the innervation of the viscera. Besides giving some output to various organs, the vagus nerve comprises between 80% and 90% of afferent nerves mostly conveying sensory information about the state of the body's organs to the central nervous system.[1]Right and left vagus nerves descend from the cranial vault through the jugular foramina, penetrating the carotid sheath between the internal and external carotid arteries, then passing posterolateral to the common carotid artery. The cell bodies of visceral afferent fibers of the vagus nerve are located bilaterally in the inferior ganglion of the vagus nerve (nodose ganglia).
The right vagus nerve gives rise to the right recurrent laryngeal nerve, which hooks around the right subclavian artery and ascends into the neck between the trachea and esophagus. The right vagus then crosses anterior to the right subclavian artery, runs posterior to the superior vena cava, descends posterior to the right main bronchus, and contributes to cardiac, pulmonary, and esophageal plexuses. It forms the posterior vagal trunk at the lower part of the esophagus and enters the diaphragm through the esophageal hiatus.
The left vagus nerve enters the thorax between left common carotid artery and left subclavian artery and descends on the aortic arch. It gives rise to the left recurrent laryngeal nerve, which hooks around the aortic arch to the left of the ligamentum arteriosum and ascends between the trachea and esophagus. The left vagus further gives off thoracic cardiac branches, breaks up into the pulmonary plexus, continues into the esophageal plexus, and enters the abdomen as the anterior vagal trunk in the esophageal hiatus of the diaphragm.
- Pharyngeal nerve
- Superior laryngeal nerve
- Superior cervical cardiac branches of vagus nerve
- Inferior cervical cardiac branch
- Recurrent laryngeal nerve
- Thoracic cardiac branches
- Branches to the pulmonary plexus
- Branches to the esophageal plexus
- Anterior vagal trunk
- Posterior vagal trunk
- Hering-Breuer reflex in alveoli[2]
Nuclei[edit]
The vagus nerve includes axons which emerge from or converge onto four nuclei of the medulla:- The dorsal nucleus of vagus nerve — which sends parasympathetic output to the viscera, especially the intestines
- The nucleus ambiguus — which gives rise to the branchial efferent motor fibers of the vagus nerve and preganglionic parasympathetic neurons that innervate the heart
- The solitary nucleus — which receives afferent taste information and primary afferents from visceral organs
- The spinal trigeminal nucleus — which receives information about deep/crude touch, pain, and temperature of the outer ear, the dura of the posterior cranial fossa and the mucosa of the larynx
Development[edit]
The motor division of the vagus nerve is derived from the basal plate of the embryonic medulla oblongata, while the sensory division originates from the cranial neural crest.Function[edit]
The vagus nerve supplies motor parasympathetic fibres to all the organs except the suprarenal (adrenal) glands, from the neck down to the second segment of the transverse colon. The vagus also controls a few skeletal muscles, notable ones being:- Cricothyroid muscle
- Levator veli palatini muscle
- Salpingopharyngeus muscle
- Palatoglossus muscle
- Palatopharyngeus muscle
- Superior, middle and inferior pharyngeal constrictors
- Muscles of the larynx (speech).
Efferent vagus nerve fibres innervating the pharynx and back of the throat are responsible for the gag reflex. In addition, 5-HT3 receptor-mediated afferent vagus stimulation in the gut due to gastroenteritis and other insults is a cause of vomiting.[3]
| This section does not cite any sources. (December 2013) (Learn how and when to remove this template message) |
- General visceral efferent (GVE) — provides parasympathetic innervation to glands of mucous membranes of the pharynx, larynx, organs in the neck, thorax, and abdomen.
- Special visceral efferent (SVE) — innervates skeletal muscles of the pharynx and larynx.
- General somatic afferent (GSA) — carries sensation from the external auditory meatus and tympanic membrane.
- General visceral afferent (GVA) — carries information from the thoracic and abdominal viscera; aortic body and arch.
- Special visceral afferent (SVA) — carries taste of the epiglottis region of the tongue.
The vagus nerve and the heart[edit]
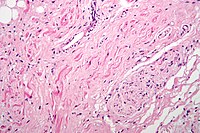
Fibers of the vagus nerve (right/bottom of image) innervate the sinoatrial node tissue (central and left of image). H&E stain.
At this location, neuroscientist Otto Loewi first demonstrated that nerves secrete substances called neurotransmitters, which have effects on receptors in target tissues. In his experiment, Loewi electrically stimulated the vagus nerve of a frog heart, which slowed the heart. Then he took the fluid from the heart and transferred it to a second frog heart without a vagus nerve. The second heart slowed down without an electrical stimulation. Loewi described the substance released by the vagus nerve as vagusstoff, which was later found to be acetylcholine. Drugs that inhibit the muscarinic receptors (anticholinergics) such as atropine and scopolamine, are called vagolytic because they inhibit the action of the vagus nerve on the heart, gastrointestinal tract, and other organs. Anticholinergic drugs increase heart rate and are used to treat bradycardia.
Physical and emotional effects[edit]
Activation of the vagus nerve typically leads to a reduction in heart rate, blood pressure, or both. This occurs commonly in the setting of gastrointestinal illness such as viral gastroenteritis or acute cholecystitis, or in response to other stimuli, including carotid sinus massage, Valsalva maneuver or pain from any cause, in particular, having blood drawn. When the circulatory changes are great enough, vasovagal syncope results. Relative dehydration tends to amplify these responses. Symptoms of irritable Bowel Syndrome are thought to cause activation of the vagus nerve with many people reporting fainting, vision disturbances and dizziness, but there has been little research into this area as it is not deemed necessary and/or life-threatening.[citation needed]Excessive activation of the vagal nerve during emotional stress, which is a parasympathetic overcompensation of a strong sympathetic nervous system response associated with stress, can also cause vasovagal syncope due to a sudden drop in cardiac output, causing cerebral hypoperfusion. Vasovagal syncope affects young children and women more than other groups. It can also lead to temporary loss of bladder control under moments of extreme fear.
Research has shown that women having had complete spinal cord injury can experience orgasms through the vagus nerve, which can go from the uterus, cervix, and, it is presumed, the vagina to the brain.[4][5]
Insulin signaling activates the adenosine triphosphate (ATP)-sensitive potassium (KATP) channels in the arcuate nucleus, decreases AgRP release, and through the vagus nerve, leads to decreased glucose production by the liver by decreasing gluconeogenic enzymes: Phosphoenolpyruvate carboxykinase, Glucose 6-phosphatase.[6][7]
Clinical significance[edit]
Vagus nerve stimulation[edit]
Main article: Vagus nerve stimulation
Vagus nerve stimulation (VNS) therapy using a pacemaker-like device implanted in the chest is a treatment used since 1997 to control seizures in epilepsy patients and has recently[needs update] been approved for treating drug-resistant cases of clinical depression.[8] A non-invasive VNS device that stimulates an afferent branch of the vagus nerve is also being developed and will soon undergo trials.[9]Clinical trials are currently underway in Antwerp, Belgium using VNS for the treatment of tonal tinnitus after a breakthrough study published in early 2011 by researchers at the University of Texas - Dallas showed successful tinnitus suppression in rats when tones were paired with brief pulses of stimulation of the vagus nerve.[10]
VNS may also be achieved by one of the vagal maneuvers: holding the breath for a few seconds, dipping the face in cold water, coughing, or tensing the stomach muscles as if to bear down to have a bowel movement.[11] Patients with supraventricular tachycardia,[11] atrial fibrillation, and other illnesses may be trained to perform vagal maneuvers (or find one or more on their own).
Vagus nerve blocking (VBLOC) therapy is similar to VNS but used only during the day. In a six-month open-label trial involving three medical centers in Australia, Mexico, and Norway, vagus nerve blocking has helped 31 obese participants lose an average of nearly 15 percent of their excess weight. A year-long 300-participant double-blind, phase II trial has begun.[12]
Vagotomy[edit]
Vagotomy (cutting of the vagus nerve) is a now-obsolete therapy that was performed for peptic ulcer disease. Vagotomy is currently being researched as a less invasive alternative weight-loss procedure to gastric bypass surgery.[13] The procedure curbs the feeling of hunger and is sometimes performed in conjunction with putting bands on patients' stomachs, resulting in average weight loss of 43% at six months with diet and exercise.[14]One serious side effect of a vagotomy is a vitamin B12 deficiency later in life — perhaps after about 10 years — that is similar to pernicious anemia. The vagus normally stimulates the stomach's parietal cells to secrete acid and intrinsic factor. Intrinsic factor is needed to absorb vitamin B12 from food. The vagotomy reduces this secretion and ultimately leads to the deficiency, which, if left untreated, causes nerve damage, tiredness, dementia, paranoia, and ultimately death.[15]
Researchers from Aarhus University and Aarhus University Hospital have demonstrated that vagotomy prevents (halves the risk of) the development of Parkinson's disease, suggesting that Parkinson's disease begins in the gastrointestinal tract and spreads via the vagus nerve to the brain.[16]
Chagasic disease[edit]
The majority of gradual devastation by neuropathy in Chagas disease is channeled to the major parasympathetic branches of the vagus nerve. Depending upon load, Chagasic vagal disease can cause megaesophagus, megacolon and cardiomyopathy.[17]History[edit]
Etymology[edit]
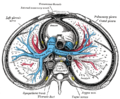
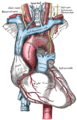
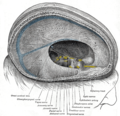
The medieval Latin word vagus means literally "wandering" (the words vagrant, vagabond, and vague come from the same root). Sometimes the branches are spoken of in the plural and are thus called vagi (/ˈveɪdʒaɪ/, US dict: vā′·jī). The vagus is also called the pneumogastric nerve since it innervates both the lungs and the stomach.Additional images[edit]
See also[edit]
This article uses anatomical terminology; for an overview, see Anatomical terminology.
- Porphyria – A rare disorder can cause seizures and damage to the vagal nerve.
- Vagovagal reflex
- Inflammatory reflex
References[edit]
- Jump up ^ Berthoud, H. R.; Neuhuber, W. L. (2000). "Functional and chemical anatomy of the afferent vagal system". Autonomic Neuroscience. 85 (1–3): 1–17. doi:10.1016/S1566-0702(00)00215-0. PMID 11189015.
- Jump up ^ Dutschmann, Mathias; Bautista, Tara G.; Mörschel, Michael; Dick, Thomas E. (2014). "Learning to breathe: Habituation of Hering–Breuer inflation reflex emerges with postnatal brainstem maturation". Respiratory Physiology & Neurobiology. 195: 44–49. doi:10.1016/j.resp.2014.02.009. ISSN 1569-9048.
- Jump up ^ Mandal, Ananya (25 September 2013). "Vomiting Mechanism". News Medical. Archived from the original on 4 January 2015. Retrieved 27 June 2015.
- Jump up ^ "Exploring the Mind-Body Orgasm". Wired. 2007-01-10.
- Jump up ^ Komisaruk BR, Whipple B, Crawford A, Liu WC, Kalnin A, Mosier K (2004). "Brain activation during vaginocervical self-stimulation and orgasm in women with complete spinal cord injury: fMRI evidence of mediation by the vagus nerves". Brain Res. 1024 (1-2): 77–88. doi:10.1016/j.brainres.2004.07.029. PMID 15451368.
- Jump up ^ Pocai, A; TK Lam; Gutierrez-Juarez R (2005). "Hypothalamic K(ATP) channels control hepatic glucose production". Nature. 434 (7036): 1026–1031. doi:10.1038/nature03439. PMID 15846348.
- Jump up ^ Pagotto, U. (2009). "Where does insulin resistance start? The brain". Diabetes Care. 32 (2): S174–S177. doi:10.2337/dc09-S305.
- Jump up ^ Nemeroff, Charles B.; et al. (2006). "VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms.". Neuropsychopharmacology. 31 (7): 1345–55. doi:10.1038/sj.npp.1301082. PMID 16641939. link
- Jump up ^ Eisenstein, Michael (2013). "Neurodevice startups target peripheral nervous system.". Nature Biotechnology. 31: 865–866. doi:10.1038/nbt1013-865.
- Jump up ^ http://www.utdallas.edu/news/2011/1/13-8021_Findings-Show-Promise-in-Battle-Against-Tinnitus_article.html
- ^ Jump up to: a b Vibhuti N, Singh; Monika Gugneja (2005-08-22). "Supraventricular Tachycardia". eMedicineHealth.com. Retrieved 2008-11-28.
- Jump up ^ Mayo Clinic. Device Blocking Stomach Nerve Signals Shows Promise in Obesity
- Jump up ^ Ulcer surgery may help treat obesity - Diet and nutrition - MSNBC.com
- Jump up ^ "Could nerve-snip spur weight loss?". CNN.com. Turner Broadcasting System. Archived from the original on 2007-07-13.
- Jump up ^ The Pernicious Anemia Society
- Jump up ^ Parkinson's disease may begin in the gut.
- Jump up ^ Córdova, E; et al. (April 2010). "Neurological manifestations of Chagas' disease.". Neurological research. 32 (3): 238–44. doi:10.1007/BF01831400. PMID 20406601.
External links[edit]
| Wikimedia Commons has media related to Nervus vagus. |
- figures/chapter_24/24-7.HTM: Basic Human Anatomy at Dartmouth Medical School
- cranialnerves at The Anatomy Lesson by Wesley Norman (Georgetown University) (X)












No comments:
Post a Comment