Sodium bicarbonate
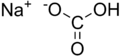 | |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Sodium hydrogen carbonate
| |||
| Other names
Baking soda, bicarb (laboratory slang), bicarbonate of soda, nahcolite
| |||
| Identifiers | |||
3D model (JSmol)
| |||
| 4153970 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.005.122 | ||
| EC Number | 205-633-8 | ||
| KEGG | |||
| MeSH | Sodium+bicarbonate | ||
PubChem CID
| |||
| RTECS number | VZ0950000 | ||
| UNII | |||
| Properties | |||
| NaHCO 3 | |||
| Molar mass | 84.0066 g mol−1 | ||
| Appearance | White crystals | ||
| Odor | Odorless | ||
| Density | |||
| Melting point | (Decomposes to sodium carbonate starting at 50 °C[7]) | ||
| Solubility | 0.02 wt% acetone, 2.13 wt% methanol @22 °C.[5] insoluble in ethanol | ||
| log P | −0.82 | ||
| Acidity (pKa) | |||
Refractive index (nD)
| nα = 1.377 nβ = 1.501 nγ = 1.583 | ||
| Structure | |||
| Monoclinic | |||
| Thermochemistry | |||
| 87.61 J/mol K | |||
Std molar
entropy (S | 102 J/mol K | ||
Std enthalpy of
formation (ΔfH | −947.7 kJ/mol | ||
Gibbs free energy (ΔfG˚)
| −851.9 kJ/mol | ||
| Pharmacology | |||
| B05CB04 (WHO) B05XA02 (WHO), QG04BQ01 (WHO) | |||
| Intravenous, oral | |||
| Hazards | |||
| Main hazards | Causes serious eye irritation | ||
| Safety data sheet | External MSDS | ||
| NFPA 704 | |||
| Flash point | Incombustible | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
| 4220 mg/kg (rat, oral)[8] | ||
| Related compounds | |||
Other anions
| Sodium carbonate | ||
Other cations
| |||
Related compounds
| |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
| Infobox references | |||
Contents
[hide]Nomenclature[edit]
Because it has long been known and is widely used, the salt has many related names such as baking soda, bread soda, cooking soda, and bicarbonate of soda. In colloquial usage, the names sodium bicarbonate and bicarbonate of soda are often truncated; forms such as sodium bicarb, bicarb soda, bicarbonate, and bicarb are common. The word saleratus, from Latin sal æratus meaning "aerated salt", was widely used in the 19th century for both sodium bicarbonate and potassium bicarbonate.The prefix bi in bicarbonate comes from an outdated naming system and is based on the observation that there is twice as much carbonate (CO3) per sodium in sodium bicarbonate (NaHCO3) as there is in sodium carbonate (Na2CO3). The modern chemical formulas of these compounds express their precise chemical compositions (which were unknown when the names sodium carbonate and sodium bicarbonate were coined) and show the same ratio the other way around: There is half as much sodium per carbonate in NaHCO3 as in Na2CO3 (Na versus Na2).
Uses[edit]
Sodium bicarbonate has a wide variety of uses.Cooking[edit]
In cooking, sodium bicarbonate, referred to as baking soda, is primarily used in baking as a leavening agent. It reacts with acidic components in batters, releasing carbon dioxide, which causes expansion of the batter and forms the characteristic texture and grain in pancakes, cakes, quick breads, soda bread, and other baked and fried foods. Acidic compounds that induce this reaction include phosphates, cream of tartar, lemon juice, yogurt, buttermilk, cocoa and vinegar. Baking soda may be used together with sourdough, which is acidic, making a lighter product with a less acid taste.[9]Heat can also by itself cause sodium bicarbonate to act as a raising agent in baking because of thermal decomposition, releasing carbon dioxide. When used this way on its own, without the presence of an acidic component (whether in the batter or by the use of a baking powder containing acid), only half the available CO2 is released. Additionally, in the absence of acid, thermal decomposition of sodium bicarbonate also produces sodium carbonate, which is strongly alkaline and gives the baked product a bitter, "soapy" taste and a yellow color. To avoid an over-acidic taste from added acid, non-acid ingredients such as whole milk or Dutch-processed cocoa are often added to baked foods.[10]
Carbon dioxide production from exposure to heat starts at temperatures above 80 °C (180 °F).[11]
- 2 NaHCO3 → Na2CO3 + H2O + CO2
Sodium bicarbonate was sometimes used in cooking green vegetables, as it gives them a bright green colour—which has been described as artificial-looking—due to its reacting with chlorophyll to produce chlorophyllin.[12] However, this tends to affect taste, texture and nutritional content, and is no longer common.[13] Baking soda is still used, though, in the traditional British mushy peas recipe for soaking the peas. It is also used in Asian and Latin American cuisine to tenderize meats. Baking soda may react with acids in food, including vitamin C (L-ascorbic acid). It is also used in breading, such as for fried foods, to enhance crispness and allow passages for steam to escape, so the breading is not blown off during cooking.
Baking powder[edit]
Baking powder, also sold for cooking, contains around 30% of bicarbonate, and various acidic ingredients which are activated by the addition of water, without the need for additional acids in the cooking medium.[14][15][16]Many forms of baking powder contain sodium bicarbonate combined with calcium acid phosphate, sodium aluminium phosphate or cream of tartar.[17] Baking soda is alkaline; the acid used in baking powder avoids a metallic taste when the chemical change during baking creates sodium carbonate.
Pest control[edit]
Sodium bicarbonate can be used to kill cockroaches. Once consumed, it causes internal organs of cockroaches to burst due to gas collection.[18]Sodium bicarbonate can be an effective way of controlling fungal growth,[19] and in the United States is registered by the Environmental Protection Agency as a biopesticide.[20]
Alkalinity/pH increase[edit]
Sodium bicarbonate can be administered to pools, spas, and garden ponds to raise the total alkalinity. This will also raise the pH level and make maintaining proper pH easier. In the event that the pH is high, sodium bicarbonate should not be used to adjust the pH.[21]Pyrotechnics[edit]
Sodium bicarbonate is one of the main components of the common "black snake" firework. The effect is caused by the thermal decomposition, which produces carbon dioxide gas to produce a long snake-like ash as a combustion product of the other main component, sucrose.Mild disinfectant[edit]
It has weak disinfectant properties,[22][23] and it may be an effective fungicide against some organisms.[24] Because baking soda will absorb musty smells, it has become a reliable method for used-book sellers when making books less malodorous.[25]Fire extinguisher[edit]
Sodium bicarbonate can be used to extinguish small grease or electrical fires by being thrown over the fire, as heating of sodium bicarbonate releases carbon dioxide.[26] However, it should not be applied to fires in deep fryers; the sudden release of gas may cause the grease to splatter.[26] Sodium bicarbonate is used in BC dry chemical fire extinguishers as an alternative to the more corrosive diammonium phosphate in ABC extinguishers. The alkaline nature of sodium bicarbonate makes it the only dry chemical agent, besides Purple-K, that was used in large-scale fire suppression systems installed in commercial kitchens. Because it can act as an alkali, the agent has a mild saponification effect on hot grease, which forms a smothering, soapy foam.Neutralization of acids and bases[edit]
Sodium bicarbonate is amphoteric, reacting with acids and bases. It reacts violently with acids, releasing CO2 gas as a reaction product. It is commonly used to neutralize unwanted acid solutions or acid spills in chemical laboratories.Medical uses and health[edit]
Sodium bicarbonate mixed with water can be used as an antacid to treat acid indigestion and heartburn.[27] Its reaction with stomach acid produces salt, water, and carbon dioxide:- NaHCO3 + HCl → NaCl + H2O + CO2(g)
HCO3 is used for treatment of hyperkalemia, as it will drive K+ back into cells during periods of acidosis.[30] Since sodium bicarbonate can cause alkalosis, it is sometimes used to treat aspirin overdoses. Aspirin requires an acidic environment for proper absorption, and the basic environment diminishes aspirin absorption in the case of an overdose.[31] Sodium bicarbonate has also been used in the treatment of tricyclic antidepressant overdose.[32] It can also be applied topically as a paste, with three parts baking soda to one part water, to relieve some kinds of insect bites and stings (as well as accompanying swelling).[33]
Sodium bicarbonate has been found to have no effect on the blood pressure of several types of rat models susceptible to salt-sensitive hypertension, in contrast with sodium chloride. This was ascribed to the high concentration of chloride, rather than the sodium content in dietary salts.[34]
Sodium bicarbonate can be used to treat an allergic reaction to plants such as poison ivy, poison oak, or poison sumac to relieve some of the associated itching.[35][better source needed]
Bicarbonate of soda can also be useful in removing splinters from the skin.[36]
Some alternative practitioners, such as Tullio Simoncini, have promoted baking soda as a cancer cure, which the American Cancer Society has warned against due to both its unproven effectiveness and potential danger in use.[37]
Sodium bicarbonate can be added to local anaesthetics, to speed up the onset of their effects and make their injection less painful.[38] It is also a component of Moffett's solution, used in nasal surgery.
It was discovered as early as the 1920s that bicarbonate caused increase in bone strength in patients who were losing calcium in their urine. In 1968 it was suggested that diets producing too much acid might put bones at risk.[39] Experiments by Anthony Sebastian of the University of California, San Francisco starting in the late twentieth century found that the body was breaking down bones and muscles to release carbonates, phosphates and ammonia, which neutralize acid. Adding bicarbonate to the diet (he used potassium bicarbonate) reduced loss of calcium in post-menopausal women, amounting to the equivalent of "an arm-and-a-leg's worth" of bone if this continued for two decades.
A wide variety of applications follows from its neutralization properties, including reducing the spread of white phosphorus from incendiary bullets inside an afflicted soldier's wounds.[40][medical citation needed]
Antacid (such as baking soda) solutions have been prepared and used by protesters to alleviate the effects of exposure to tear gas during protests.[41][not in citation given][42]
Personal hygiene[edit]
Toothpaste containing sodium bicarbonate has in several studies been shown to have a better whitening[43][43][44][45] and plaque removal effect[46][47] than toothpastes without it.Sodium bicarbonate is also used as an ingredient in some mouthwashes. It has anticaries and abrasive properties.[48] It works as a mechanical cleanser on the teeth and gums, neutralizes the production of acid in the mouth, and also acts as an antiseptic to help prevent infections.[citation needed]
Sodium bicarbonate in combination with other ingredients can be used to make a dry or wet deodorant.[49][50]
Sodium bicarbonate may be used as a buffering agent, combined with table salt, when creating a solution for nasal irrigation.[51]
It is used in eye hygiene to treat blepharitis. This is done by addition of a teaspoon of sodium bicarbonate to cool water that was recently boiled, followed by gentle scrubbing of the eyelash base with a cotton swab dipped in the solution.[52]
Veterinary uses[edit]
Sodium bicarbonate is used as a cattle feed supplement, in particular as a buffering agent for the rumen.[53]In sports[edit]
Small amounts of sodium bicarbonate have been shown to be useful as a supplement for athletes in speed-based events, such as middle-distance running, lasting from about one to seven minutes.[54][55] However, overdose is a serious risk because sodium bicarbonate is slightly toxic;[56] and gastrointestinal irritation is of particular concern.[55] Additionally, this practice causes a significant increase in dietary sodium.[citation needed]Cleaning agent[edit]
Sodium bicarbonate is used in a process for removing paint and corrosion called sodablasting; the process is particularly suitable for cleaning aluminium panels which can be distorted by other types of abrasives.A paste made from baking soda with minimal water is recommended by a manufacturer as a gentle scouring powder,[26] and is useful in removing surface rust, as the rust forms a water-soluble compound when in a concentrated alkaline solution;[57] cold water should be used, as hot water solutions can corrode steel. [58] Sodium bicarbonate attacks the thin unreactive protective oxide layer that forms on aluminium, making it unsuitable for cleaning this otherwise very reactive metal.[59] A solution in warm water will remove the tarnish from silver when the silver is in contact with a piece of aluminium foil.[59][60] Baking soda is commonly added to washing machines as a replacement for water-softener and to remove odors from clothes. Sodium bicarbonate is also effective in removing heavy tea and coffee stains from cups when diluted with warm water. Also, baking soda can be used as a multi purpose odor remover.[61]
During the Manhattan Project to develop the nuclear bomb in the early 1940s the chemical toxicity of uranium was an issue. It was found that uranium oxides stick very well to cotton cloth, and did not wash out with soap or laundry detergent. However, the uranium would wash out with a 2% solution of sodium bicarbonate. Clothing can become contaminated with toxic dust of depleted uranium (DU), which is very dense, and hence used for counterweights in a civilian context, and in armour-piercing projectiles. DU is not removed by normal laundering; washing with about 6 ounces (170 g) of baking soda in 2 gallons (7.5 l) of water will help to wash it out.[62]
Chemistry[edit]
Sodium bicarbonate is an amphoteric compound. Aqueous solutions are very mildly alkaline due to the formation of carbonic acid and hydroxide ion:- HCO−
3 + H2O → H
2CO
3 + OH−
- NaHCO3 + HCl → NaCl + H2CO3
- H2CO3 → H2O + CO2(g)
- NaHCO3 + CH3COOH → CH3COONa + H2O + CO2(g)
- NaHCO3 + NaOH → Na2CO3 + H2O
2. This reaction is used to test for the presence of carboxylic groups in protein.[citation needed]
Thermal decomposition[edit]
Above 50 °C, sodium bicarbonate gradually decomposes into sodium carbonate, water and carbon dioxide. The conversion is fast at 200 °C:[63]- 2 NaHCO3 → Na2CO3 + H2O + CO2
- Na2CO3 → Na2O + CO2
History[edit]
In 1791 French chemist Nicolas Leblanc produced sodium carbonate, also known as soda ash. In 1846 two New York bakers, John Dwight and Austin Church, established the first factory in the United States to produce baking soda from sodium carbonate and carbon dioxide.[64]Saleratus, potassium or sodium bicarbonate, is mentioned in the novel Captains Courageous by Rudyard Kipling as being used extensively in the 1800s in commercial fishing to prevent freshly caught fish from spoiling.[65]
Production[edit]
NaHCO3 is mainly prepared by the Solvay process, which is the reaction of sodium chloride, ammonia, and carbon dioxide in water. Calcium carbonate is used as the source of CO2 and the resultant calcium oxide is used to recover the ammonia from the ammonium chloride. The product shows a low purity (75%). Pure product is obtained from sodium carbonate, water, and carbon dioxide as reported in one of the following reactions. It is produced on the scale of about 100,000 tonnes/year (as of 2001).[66]NaHCO3 may be obtained by the reaction of carbon dioxide with an aqueous solution of sodium hydroxide. The initial reaction produces sodium carbonate:
- CO2 + 2 NaOH → Na2CO3 + H2O
- Na2CO3 + CO2 + H2O → 2 NaHCO3
- Na2CO3 + CO2 + H2O → 2 NaHCO3



No comments:
Post a Comment